<<< >>>
{477}
{478}
{479}
Birds
Waterfowl and Other Aquatic Birds
GEESE, SWANS, AND DUCKS
 Geese
Geese
GREYLAG GOOSE (Anser anser)
IDENTIFICATION: A dark gray goose with fine silvery-white feather patterning; the wild ancestor of domestic geese. DISTRIBUTION: Northern and central Eurasia, from Iceland to northeast China. HABITAT: Variable, including marshes, swamps, lakes, lagoons. STUDY AREAS: Konrad-Lorenz Institute, Grunau, Austria; Max-Planck Institute, Seewiesen, Germany; Worlitzer Park, Dessau, Germany; subspecies
A.a. anser.
Social Organization
Greylag Geese usually associate in flocks containing a complex mixture of pairs, families with offspring, single birds, and subgroups of juveniles. Following the breeding season, migratory flocks sometimes contain thousands of birds. The mating system generally involves long-term, monogamous pair-bonding.

A Greylag gander pair performing a synchronized duet of "rolling" calls. In this and other species, both males in a homosexual pair perform mutual or typically "male" activities rather than one bird adopting a "male" role and the other a "female" role.
Description
Behavioral Expression: Homosexual pairs made up of two ganders are a prominent form of pair-bonding in Greylag Geese. Male couples are stable and long-lasting: some have been documented as persisting for more than fifteen years, and most homosexual pairs (like heterosexual ones) are probably lifelong partnerships (Greylag Geese can live to be more than 20 years old). "Widower" ganders may even exhibit signs of "grief," becoming despondent and defenseless upon the loss of their male partner. Most heterosexual pairs are also lifelong (and partners {480}
grieve the loss of their mates), but in many cases gander pairs are actually more closely bonded than male-female pairs, due in part to the intensity of their displays. One of these is the TRIUMPH CEREMONY, a pair-bonding behavior in which the two partners approach each other with extended necks and spread wings while making loud gabbling calls. Gander pairs spend significantly more time in this activity than do heterosexual pairs. They are also generally more vocal than male-female pairs — they often utter PRESSED CACKLING calls (rapid syllables produced with a high-pressure airstream) together in a cheek-to-cheek position and may even perform extended duets with ROLLING calls (deeper and louder notes).
Paired ganders also sometimes engage in courtship and sexual behavior with one another. Pair-bonding is often initiated with the BENT-NECK DISPLAY, in which one male approaches and follows another with a distinct "kink" in his neck, his bill pointing downward. As a prelude to mating, both males perform aquatic displays such as NECK-DIPPING or NECK-ARCHING, in which the head is dipped below the surface while the neck is held in an elegant curve, its feathers ruffled to reveal their distinctive patterning. Following these displays, one male may mount the other as in heterosexual copulation. If there is a size difference between the two males, often the larger male mounts the smaller one. If the two ganders are equal in size, either bird may mount the other, and they often exchange positions when they copulate on different days. Following mating, the male who mounted his partner performs a display in which he lifts his head up and arches his folded wings almost vertically above his back. Sometimes, during homosexual activity one male may "masturbate" by mounting a log or some other object (a common form of masturbation in birds). In addition, a third bird — either male or female — occasionally joins a homosexual pair in their courtship activities, and may even be mounted by one of the ganders. In all cases, though, the {481}
concluding display takes place between the members of the male pair rather than with the third bird. Some gander pairs do not regularly engage in full mounting behavior, in part because both males prefer to mount each other without either one permitting himself to be mounted.
Gander pairs often assume a powerful, high-ranking position within their flock, owing to their superior strength and courage. They are notably more aggressive than heterosexual pairs, frequently threatening, charging, chasing, and jointly attacking predators as well as other geese (especially unpaired males) and often appear to "terrorize" other birds. Paradoxically, each individual gander in a homosexual pair is significantly less aggressive than a male in a heterosexual pair — it is their combined strength that gives the couple its advantage. Homosexual pairs also differ from heterosexual ones in spending significantly more time on the periphery of the flock or away from it, especially during the spring breeding season. This, combined with the gander pair's greater vigilance behavior (as well as the pair's aggressiveness), has led some researchers to suggest that homosexual pairs may act as "guardians" for the flock as a whole. Sometimes a female is attracted to a gander pair — perhaps because of their strength and high standing — and tries to establish a bond with one or both of them. Often the males simply ignore such a female, but in some cases she is allowed to join them to form a trio. When this happens, one or both ganders may copulate with the female, although their homosexual bond usually remains primary. The trio may raise a family together, with the two ganders often searching for a nest site together and jointly defending their eggs and goslings. Occasionally, three ganders bond with each other as a same-sex trio, which may also later be joined by a female to form a "quartet"; again, goslings can be raised by all four birds together.
Although most gander pairs are stable partnerships, occasionally one or both birds may behave antagonistically toward his partner. Fights sometimes erupt when one male tries to mount the other, while occasionally the aggression aimed at an intruder is turned back on one of the partners. Bonded ganders (especially in trios) may also become "jealous" of the attentions their partner shows toward another bird. Some gander pairs are incestuous, as brothers may form long-term homosexual bonds. In addition, interspecies same-sex pairs also occur, for example between Greylag Geese and Mute Swans. Like Greylag-only gander pairs, these partnerships are long-lasting and distinguish themselves by their aggressiveness, with the two males frequently defending their territory against intruders.
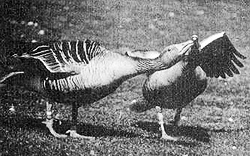
Two pair-bonded male Greylag Geese performing the "triumph ceremony"
Frequency: Homosexual couples constitute a significant proportion of pairs in Greylag Geese: an average of 14 percent of pairs in some populations are same-sex, and in some years this proportion can be even higher, with more than 20 percent of all pair-bonds consisting of ganders.

A Greylag gander mounting his male partner
Orientation: Some Greylag males in gander pairs are exclusively homosexual, since they remain in a monogamous same-sex pair-bond for their entire lives (or re-pair with another gander on the death of their partner). Other males, however, are bisexual: some copulate with a female while remaining primarily bonded to a {482}
male (as described above), while others are involved in bisexual trios. Still other males alternate or switch between female and male partners over their lives — for example, ganders in heterosexual pairs sometimes find a male partner after the death of their mate. More than half of all widowers re-pair with a bird of the opposite sex, less than a third remain single, while the remainder form homosexual bonds.
Nonreproductive and Alternative Heterosexualities
Several variations on the monogamous, lifelong pair-bond occur in this species. Divorce occasionally happens: in some populations as many as a quarter of all females, for example, may abandon their mates and find a new gander, and overall, 5-8 percent of pairs divorce. Greylag Geese also sometimes form polygamous heterosexual trios, in which bonding occurs primarily between birds of the opposite sex — two males with a female or, more rarely, two females with a male. In addition, some families foster-parent chicks by combining broods with another family, while widowed ganders occasionally adopt goslings. Birds in heterosexual pairs may engage in promiscuous courtship and mating. Ganders sometimes try to mount females other than their mate, while females may pursue other males — much to the consternation of their mates, who often try to physically prevent them from engaging in "extramarital" activities. Although Greylag Geese become sexually mature in their third year, some one-year-olds form pair-bonds and even engage in courtship and sexual activity long before they begin breeding. Like homosexual pairs, heterosexual associations may also occur between related birds (especially parent-offspring), or birds of different species (e.g., with Canada Geese). However, sibling pairings are much less common among birds of the opposite sex.
Sources
(* asterisked references discuss homosexuality/transgender)
Ens, B. J., S. Choudhury, and J. M. Black (1996) "Mate Fidelity and Divorce in Monogamous Birds." In J. M. Black, ed., Partnerships in Birds: The Study of Monogamy, pp. 344-401. Oxford: Oxford University Press.
Huber, R. (1988) "Sex-Specific Behavior in Greylag Geese, Anser anser L." Texas Journal of Science 40:107-9.
* Huber, R., and M. Martys (1993) "Male-Male Pairs in Greylag Geese (Anser anser)." Journal fur Ornithologie 134:155-64.
* Lorenz, K. (1991) Here Am I — Where Are You? The Behavior of the Greylag Goose. New York: Harcourt Brace Jovanovich.
* --- (1979) The Year of the Greylag Goose. New York: Harcourt Brace Jovanovich.
Olsson, H. (1978) "Probable Polygamy in the Greylag Goose, Anser anser, and an Instance of Combined Broods." Var Fagelvarld 37:257-58.
* Schonfeld, M. (1985) "Beitrag zur Biologie der Schwane: 'Mannchenpaar' zwischen Graugans und Hock-erschwan [Contribution to the Biology of Swans: 'Male Pairing' Between a Greylag Goose and a Mute Swan]." Der Falke 32:208.
{483}
 Geese
Geese
CANADA GOOSE (Branta canadensis)
IDENTIFICATION: A brown-plumaged goose with a distinctive black neck and white cheek patch; varies widely in size, from 2-24 pounds. DISTRIBUTION: Mostly throughout North Amerca. HABITAT: Lakes, rivers, marshes, meadows, and tundra. STUDY AREAS: Horicon Marsh Wildlife Refuge, Wisconsin; Holkham Park, England; in captivity in Ithaca, New York; subspecies B.c. interior, the Hudson Bay Canada Goose, and B.c. canadensis, the Atlantic Canada Goose.
SNOW GOOSE (Anser caerulescens)
IDENTIFICATION: A pinkish-red-billed goose with two major color phases: all-white and "blue" (grayish plumage with a white head and neck). DISTRIBUTION: Alaska and north-central Canada, northwestern Greenland; winters in southern United States and northern Mexico. HABITAT: Tundra, marshes, floodlands. STUDY AREAS: La Perouse Bay, Churchill, Manitoba, Canada; Carver Park, Minnesota; subspecies A.c. caerulescens, the Lesser Snow Goose.
Social Organization
Snow Geese are extremely gregarious and nest in dense colonies that can number in the thousands of birds; Canada Goose breeding grounds are generally less dense. In both species, birds usually pair in long-term, monogamous bonds (albeit with a number of variations — see below), and outside of the mating season they gather in large flocks.
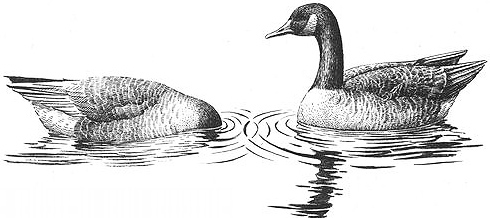
Courtship between a pair of female Canada Geese: the "head-dipping" display
Description:
Behavioral Expression: In Canada Geese, two birds of the same sex sometimes form a pair-bond. Both male and female homosexual pairs occur, and the partners may be either adults or juveniles; homosexual pair-bonds often persist for many years (as do heterosexual ones). Courtship behavior in the form of HEAD-DIPPING is frequently a part of such bonds: in this display, one bird ritually splashes water over the back of its head and neck by dipping its head deep into the water and then lifting it back up. In heterosexual contexts, this is often a prelude to copulation, but homosexual copulation is not a prominent feature of same-sex pairs. One exception involves trios: occasionally a bond forms between three birds — two females {484}
and one male — and sometimes one of the females will mount and copulate with the other female. Some lesbian pairs try to raise a family: one female in a homosexual pair, for example, built a nest and laid eggs while her partner stood guard, then the other female built her own nest next to the first and also laid eggs. None of the eggs hatched, however, because the females constantly rolled the eggs (which were probably not fertile in any case) back and forth between their nests and broke all of them.
More successful lesbian parenting occurs in Snow Geese. Pair-bonds between females are strong: when one member of the pair is absent from her mate, the other begins loudly calling to her until she returns. The pair builds a single nest in which each female lays eggs; as a result, such nests may have SUPERNORMAL CLUTCHES containing double the number of eggs found in heterosexual nests (8 eggs versus 4-5). Both birds take turns incubating the eggs (in heterosexual pairs, the male does not incubate). Since one or both females sometimes copulate with males, some of their eggs may be fertile. When they hatch, both females raise the goslings, including defending them against intruders and predators (such as Herring Gulls) by standing over them with cupped wings. Male homosexual pairs are not found in Snow Geese, although occasionally a cross-species pairing does develop between a male Snow Goose and a male Canada Goose. The two birds become constant companions, following one another and roosting close together, although nest-building and copulation do not usually take place. However, same-sex mounting does sometimes occur between male Snow Geese who take part in heterosexual "gang rapes." In this species, males often sexually harass females, chasing them and forcing them to copulate. In some cases, other males gather together in large "spectator" groups — sometimes containing as many as 20-80 males — to watch and perhaps even join in. Occasionally, one male mounts another male as part of the group sexual activity that ensues.
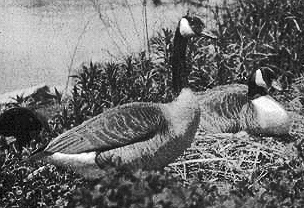
A mated pair of female Canada Geese
Frequency: In Canada Geese, up to 12 percent of pairs in some (semiwild) populations are homosexual. The proportion is much smaller in Snow Geese: about 1 in 200 nests belongs to a pair of females. Approximately 4 percent of all mountings during Snow Goose rape attempts are between males.
{485}
Orientation: In one study of Canada Geese, 18 percent of the males formed homosexual pair-bonds while 6-12 percent of females did. Some birds in same-sex pairs appear to "prefer" their homosexual association, even if they have the opportunity for heterosexual interactions. In one case, a male harassed a female who was part of a long-lasting lesbian pair and separated her from her companion, mating with her. However, the next year she returned to her female partner and their pair-bond resumed. On the other hand, some birds have a preference for heterosexual pairings: many males remain unpaired if there are no available females rather than forming homosexual pair-bonds with each other. In Snow Geese, females in homosexual pairs may be functionally bisexual — they sometimes copulate with up to three different males to fertilize their eggs — although their same-sex pair-bond remains primary. Males who mount other males are otherwise primarily heterosexual, since most are paired with females and the majority of their sexual interactions are probably not with males.
Nonreproductive and Alternative Heterosexualities
As mentioned above, heterosexual rape is common in Snow Geese: during some mating seasons, each female is subjected to a rape attempt every five days (on average). Females are occasionally successful in thwarting such attacks, but males who rape can be very aggressive and may attack in groups. Sometimes the female's mate can successfully chase an intruder off, but often he is not around to defend her because he is also raping another female. Significantly, most rapes are nonreproductive: more than 80 percent of all rape attempts are directed toward females that are nonfertilizable, such as incubating birds, and only about 2 percent of goslings are actually fathered this way. Rape is much less common among Canada Geese. However, ganders frequently harass and attack neighboring females when their mates are gone, often leading to abandonment of their eggs — as many as one-quarter of all nests may end up being deserted this way.
Several other variations on the heterosexual nuclear family occur in these species. Although most male-female pairs remain together for life, divorce and remating do occasionally take place in both Canada and Snow Geese. In addition, although most Snow Goose families remain together until the next breeding season, in some populations as many as 20 percent of the families split or break up before that time, usually because of separation of juveniles. Polygamous, heterosexual trios consisting of one male and two females sometimes form in Canada Geese (these differ from the bisexual trios described above in that the females are not bonded to each other). Some birds pair outside of their species, and Snow and Canada Geese may in fact mate with each other.
CRÈCHES or combined broods — containing as many as 60 young — are sometimes found in Canada Geese, attended by one or several heterosexual pairs. In addition, families often "trade" goslings, caring for young other than their own on either a temporary or permanent basis. Up to 46 percent of Canada Goose broods and at least 13 percent of Snow Goose broods may contain adopted young, and over 60 percent of Canada broods in some populations experience a loss and/or gain of goslings due to adoption. Egg "adoption" {486}
is also common in Snow Geese because females often lay eggs in nests other than their own: 15-22 percent of all nests contain such eggs (although in some colonies more than 80 percent of nests may be affected), and more than 5 percent of all goslings are raised by a female other than their biological mother. Females that lay eggs in others' nests are often aided by their mates, who distract the nest-owning gander by acting as a decoy for him to attack, allowing the female to approach the nest and lay her eggs. Sometimes the intruding female actually helps with building or repairing the nest; for her part, the nest-owning female often actively adopts foreign eggs that have not been laid directly in the nest by rolling them into her own clutch.
Snow Goose females also occasionally "abandon" their eggs by laying in what are known as DUMP NESTS, which contain large numbers of unincubated eggs from many different females. Abandonment of nests may also be triggered by the stresses of reproduction: females can lose up to a third of their body mass while incubating, and some individuals desert their clutches or even starve on the nest as a result of such hardships. Most Snow Goose nesting colonies also have a nonbreeding flock on their peripheries. In some years, the proportion of nonbreeding adults is sizable — as much as 40 percent of the population — and occasionally an entire colony will forgo breeding (for example if the weather is particularly adverse). Many Canada Goose heterosexual pairs are nonbreeding as well: in some populations, for example, more than a quarter of all male-female pairs do not procreate, although they may copulate frequently. In fact, some nonbreeders have sexual activity rates that are almost twice as high as pairs that do reproduce.
Sources
(* asterisked references discuss homosexuality/transgender)
* Allen, A. A. (1934) "Sex Rhythm in the Ruffed Grouse (Bonasa umbellus Linn.) and Other Birds." Auk 51:180-99.
Ankney, C. D., and C. D. MacInnes (1978) "Nutrient Reserves and Reproductive Performance of Female Lesser Snow Geese." Auk 95:459-71.
* Collias, N. E., and L. R. Jahn (1959) "Social Behavior and Breeding Success in Canada Geese (Branta canadensis) Confined Under Semi-Natural Conditions." Auk 76:478-509.
* Conover, M. R. (1989) "What Are Males Good For?" Nature 342:624-25.
Cooke, E., and D. S. Sulzbach (1978) "Mortality, Emigration, and Separation of Mated Snow Geese." Journal of Wildlife Management 42:271-80.
Cooke, F., M. A. Bousfield, and A. Sadura (1981) "Mate Change and Reproductive Success in the Lesser Snow Goose." Condor 83:322-27.
* Diamond, J. M. (1989) "Goslings of Gay Geese." Nature 340:101.
Ewaschuk, E., and D. A. Boag (1972) "Factors Affecting Hatching Success of Densely Nesting Canada Geese." Journal of Wildlife Management 36:1097-106.
* Grether, G. F., and A. M. Weaver (1990) "What Are Sisters Good For?" Nature 345:392.
* Klopman, R. B. (1962) "Sexual Behavior in the Canada Goose." Living Bird 1:123-29.
Lank, D. B., P. Mineau, R. F. Rockwell, and F. Cooke (1989) "Intraspecific Nest Parasitism and Extra-Pair Copulation in Lesser Snow Geese." Animal Behavior 37:74-89.
Luekpe, K. (1984) "A Strange Goose: Canada-Snow Hybrid?" Passenger Pigeon 46:92.
MacInnes, C. D., R. A. Davis, R. N. Jones, B. C. Lieff, and A. J. Pakulak (1974) "Reproductive Efficiency of McConnell River Small Canada Geese." Journal of Wildlife Management 38:686-707.
Martin, K., F. G. Cooch, R. F. Rockwell, and F. Cooke (1985) "Reproductive Performance in Lesser Snow Geese: Are Two Parents Essential?" Behavioral Ecology and Sociobiology 17:257-63.
* Mineau, P., and F. Cooke (1979) "Rape in the Lesser Snow Goose." Behavior 70:280-91.
Nastase, A. J., and D. A. Sherry (1997) "Effect of Brood Mixing on Location and Survivorship of Juvenile Canada Geese." Animal Behavior 54:503-7. {487}
Prevett, J. P. and C. D. MacInnes (1980) "Family and Other Social Groups in Snow Geese." Wildlife Monographs 71:1-46.
* Quinn, T. W., J. C. Davies, F. Cooke, and B. N. White (1989) "Genetic Analysis of Offspring of a Female-Female Pair in the Lesser Snow Goose (Chen c. caerulescens)." Auk 106:177-84.
* Starkey, E. E. (1972) "A Case of Interspecific Homosexuality in Geese." Auk 89:456-57.
Syroechkovsky, E. V. (1979) "Podkladyvaniye byelymi gusyami yaits v chuzhiye gnyezda [The Laying of Eggs by White Geese into Strange Nests]." Zoologichesky Zhurnal 58:1033-41.
Williams, T. D. (1994) "Adoption in a Precocial Species, the Lesser Snow Goose: Intergenerational Conflict, Altruism, or a Mutually Beneficial Strategy?" Animal Behavior 47:101-7.
Zicus, M. C. (1984) "Pair Separation in Canada Geese." Wilson Bulletin 96:129-30.
 Swans
Swans
BLACK SWAN (Cygnus atratus)
IDENTIFICATION: The only swan with fully black plumage; wing feathers are white, bill is bright red, and the neck is especially long. DISTRIBUTION: Australia, Tasmania, New Zealand. HABITAT: Lakes, lagoons, swamps, bays, floodlands. STUDY AREAS: Lake George and Lake Bathurst, New South Wales, Australia; in captivity at the Division of Wildlife Research, Canberra, Australia.
MUTE SWAN (Cygnus olor)
IDENTIFICATION: A large swan (up to 33 pounds) with a black knob at the base of its reddish orange bill (less prominent in females). DISTRIBUTION: Europe and temperate Asia. HABITAT: Marshes, ponds, lakes, slow-moving rivers, lagoons, coastal areas. STUDY AREAS: Abbotsbury (Dorset) and Rainworth Lodge (Notts), England; Renfrewshire, Scotland.
Social Organization
Black Swans sometimes flock by the thousands and usually form mated pairs (although with numerous variations — see below) that nest either colonially or in separate territories. Mute Swans also generally develop long-term, monogamous {488}
bonds and nest in widely spaced territories, although some pairs form nesting colonies. Outside of the breeding season, they often associate in flocks.
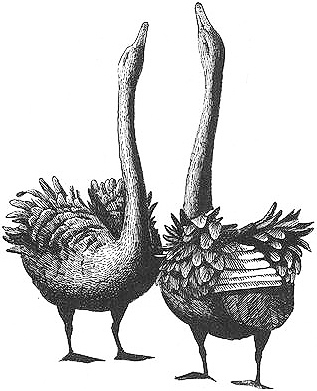
A homosexual pair of male Black Swans performing the "greeting ceremony"
Description
Behavioral Expression: Some male Black Swans form stable, long-lasting homosexual pairs. Like heterosexual mates, same-sex partners often remain together for many years. The two males frequently perform the GREETING CEREMONY, a pair-bonding display that helps solidify and reinforce their partnership: the birds face one another, raise their wings (sometimes flapping them to expose the white feathers), and call repeatedly while extending their necks and lifting their bills up. Males in homosexual pairs also perform a courtship behavior known as HEAD-DIPPING. In this display — a prelude to copulation — the two birds repeatedly immerse first the head, then the neck, and finally the body in a wavelike fashion, sometimes for extended periods of 20-25 minutes. This can lead to homosexual mounting, although if one male does not want to participate in sexual activity he may respond aggressively to his partner's overtures.
Male pairs of Black Swans fiercely defend territories that, during the mating season, are often significantly larger than those of heterosexual pairs. Because two males are able to pool their strength, they are more successful at chasing away other swans and can often annex a major portion of a pond (1,500-3,300 square feet) into their territory. In contrast, heterosexual pairs are often relegated to less favorable nesting areas and smaller territories (15-60 square feet). Homosexual pairs are also successful parents, acquiring nests and eggs in two different ways. Some male pairs associate temporarily with a female, building a nest together, mating with her, and then chasing her away once the eggs are laid, after which they begin parenting as a male couple. Other homosexual pairs chase heterosexual pairs from their nests and adopt the eggs that have already been laid. The two males incubate the clutch, hatch the eggs, and raise the chicks together. In fact, homosexual pairs are often more successful than heterosexual ones at raising chicks, in part because they have access to the best nesting sites and the largest territories, and probably because they also share incubation duties more equally. On average, 80 {489}
percent of homosexual parenting efforts are successful, compared to only about 30 percent of heterosexual ones.
Both male and female homosexual pairs occur in Mute Swans. In female pairs, both birds build a nest, lay eggs (which are usually infertile), and incubate the eggs. Sometimes one female stands guard over both the nest and her mate (as does the male in heterosexual pairs) and defends their territory. If their nest is disturbed by intruders, the females may begin a second nest and lay a new clutch of eggs, while still attending to the first as best they can. Male pairs also annually build nests together on which they take turns sitting, although unlike Black Swans they do not acquire any eggs. Male Mute Swans also sometimes form homosexual pair-bonds with other species, including Trumpeter Swans (Cygnus buccinator) and Greylag Geese.

The earliest photographic record of animal homosexuality: a pair of male Mute Swans photographed in 1923 on the nest they built together in Scotland. A female pair in the same species was first observed in 1885.
Frequency: Overall, male couples constitute 5-6 percent of all pairings in Black Swans; in a given year, an average of 13 percent of the birds are in homosexual pair-bonds, and sometimes this proportion is as high as 20 percent. Homosexual parents account for 20-25 percent of all successful families. Same-sex bonds probably occur only sporadically in Mute Swans.
Orientation: Many Black and Mute Swans in same-sex pairs are probably exclusively homosexual, since they do not engage in heterosexual copulation or pairing and usually ignore unpaired birds of the opposite sex. However, some male pairs of Black Swans — while primarily homosexual — form short-lived bisexual trios in order to mate with females and thereby father their own offspring.
Nonreproductive and Alternative Heterosexualities
Populations of both Mute and Black Swans contain large proportions of nonbreeding birds. More than half of all Mute Swans are nonbreeders (as much as 89 percent of some populations), often gathering into their own flocks separate from breeding pairs. Many birds nest only once or twice during their entire lifetimes (which can last for 15-20 years), and a few never breed. Overall, only about a fifth of all Black Swans nest in any year, and in some populations more than 90 percent of the adults do not breed. Young, sexually mature Black Swans may remain with their parents and delay their breeding for many years (as long as three to eight years in some cases). On occasion, such a youngster will form an incestuous pair-bond with its parent: male Swans have been known to mate with their mother on the death of their father. Brother-sister and parent-offspring matings also occur in Mute Swans, as do heterosexual pairings with other species of swans (such as bewick's, whistling, whooper, and Trumpeter) and geese (e.g., Snow, Canada, and Greylag). In fact, Black and Mute Swans may pair with each other, and trios of a male Black with two female Mute Swans have also been observed. Heterosexual trios within the same species are also common: about 14 percent of all bonds in Black Swans involve two males with a female, while Mute Swan trios are usually made up of two females with a male. {490}
In addition to such polygamous associations, several other alternative family arrangements occur. "Foster parenting" or adoption takes place frequently among Black Swans (and occasionally in Mute Swans). In some colonies, more than two-thirds of all cygnets are raised in broods that combine offspring from 2-4 families (and occasionally from as many as 30 different families). Such BROOD AMALGAMATIONS — which may have up to 40 youngsters — are attended by a single pair of adults, who are not necessarily the biological parents of any of the cygnets. Adoption also occasionally occurs when adults "steal" eggs laid near their nest by other birds, rolling them into their own nest. Single parenting is a prominent feature of Black Swan social life: often a male or female deserts its mate during incubation, and in some colonies the majority of nests are attended by single parents. Occasionally, a pair "separates" rather than divorces, with one bird taking the newly hatched young while the other remains to incubate the rest of the eggs. Among Mute Swans, the divorce rate is 3-10 percent of all pairs, and about a fifth of all birds have two to four mates during their lifetime. Some Mute Swans are nonmonogamous, courting or mating with another bird while remaining paired with their partner; some of this activity may involve REVERSE copulations (in which the female mounts the male). Many within-pair copulations are nonprocreative, since most pairs mate far more often than is required for fertilization of the eggs. Swans also sometimes engage in behaviors that are counterreproductive. A third of all Black Swan eggs, for example, are lost through abandonment of the nest by the parent (s), while 3 percent of Mute Swan parents desert their nests, and birds often attack and even kill youngsters that stray into their territory. Eggs are sometimes also destroyed during territorial disputes, and adult birds may be killed as a direct result of such attacks as well (accounting for 3 percent of all deaths).
Sources
(* asterisked references discuss homosexuality/transgender)
Bacon, P. J., and P. Andersen-Harild (1989) "Mute Swan." In 1. Newton, ed., Lifetime Reproduction in Birds, pp. 363-86. London: Academic Press.
Braithwaite, L. W. (1982) "Ecological Studies of the Black Swan. IV. The Timing and Success of Breeding on Two Nearby Lakes on the Southern Tablelands of New South Wales." Australian Wildlife Research 9:261-75.
* --- (1981) "Ecological Studies of the Black Swan. III. Behavior and Social Organization." Australian Wildlife Research 8:135-46.
* --- (1970) "The Black Swan." Australian Natural History 16:375-79.
Brugger, C., and M. Taborsky (1994) "Male Incubation and Its Effect on Reproductive Success in the Black Swan, Cygnus atratus." Ethology 96:138-46.
Ciaranca, M. A., C. C. Allin, and G. S. Jones (1997) "Mute Swan (Cygnus olor)." In A. Poole and F. Gill, eds., The Birds of North America: Life Histories for the 21st Century, no. 273. Philadelphia: Academy of Natural Sciences; Washington, D.C.: American Ornithologists' Union.
Dewer, J. M. (1942) "Menage a Trois in the Mute Swan." British Birds 30:178.
Huxley, J. S. (1947) "Display of the Mute Swan." British Birds 40:130-34.
* Kear, J. (1972) "Reproduction and Family Life." In P. Scott, ed., The Swans, pp. 79-124. Boston: Houghton Mifflin.
* Low, G. C. and Marquess of Tavistock (1935) "The Extent to Which Captivity Modifies the Habits of Birds." Bulletin of the British Ornithologists' Club 55:144-54.
Mathiasson, S. (1987) "Parents, Children, and Grandchildren — Maturity Process, Reproduction Strategy, and Migratory Behavior of Three Generations and Two Year-Classes of Mute Swans Cygnus olor." In M. O. G. Eriksson, ed., Proceedings of the Fifth Nordic Ornithological Congress, 1985, pp. 60-70. Acta Regiae Societatis Scientiarum et Litterarum Gothoburgensis Zoologica no. 14. Goteborg: Kungl. Vetenskaps- och Vitterhets-Samhallet. {491}
Minton, C. D. T. (1968) "Pairing and Breeding of Mute Swans." Wildfowl 19:41-60.
* O'Brien, R. M. (1990) "Black Swan, Cygnus atratus." In S. Marchant and P. J. Higgins, eds., Handbook of Australian, New Zealand, and Antarctic Birds, vol. 1, pp.1178-89. Melbourne: Oxford University Press.
Ogilvie, M. A. (1972) "Distribution, Numbers, and Migration." In P. Scott, ed., The Swans, pp. 29-55. Boston: Houghton Mifflin.
Rees, E. C., P. Lievesley, R. A. Pettifor, and C. Perrins (1996) "Mate Fidelity in Swans: An Interspecific Comparison." In J. M. Black, ed., Partnerships in Birds: The Study of Monogamy, pp. 118-37. Oxford: Oxford University Press.
* Ritchie, J. P. (1926) "Nesting of Two Male Swans." Scottish Naturalist 159:95.
* Schonfeld, M. (1985) "Beitrag zur Biologie der Schwane: 'Mannchenpaar' zwischen Graugans und Hock-erschwan [Contribution to the Biology of Swans: 'Male Pairing' Between a Greylag Goose and a Mute Swan]." Der Falke 32:208.
Sears, J. (1992) "Extra-Pair Copulation by Breeding Male Mute Swan." British Birds 85:558-59.
* Whitaker, J. (1885) "Swans' Nests." The Zoologist 9:263-64.
Williams, M. (1981) "The Demography of New Zealand's Cygnus atratus Population." In G. V. T. Matthews and M. Smart, eds., Proceedings of the 2nd International Swan Symposium (Sapporo, Japan), pp. 147-61. Slimbridge: International Waterfowl Research Bureau.
 Ducks
Ducks
MALLARD DUCK (Anas platyrhynchos)
IDENTIFICATION: A familiar duck with a blue wing patch, an iridescent green head and white collar in males, and brown, mottled plumage in females. DISTRIBUTION: Throughout the Northern Hemisphere; Australia and New Zealand. HABITAT: Wetlands. STUDY AREAS: J. Rulon Miller Wildlife Refuge, McDonogh, New Jersey; Haren and Middleburg, the Netherlands; Augsburg, Germany, and the Max-Planck Institute, Seewiesen, Germany; Delta Waterfowl Research Station, Lake Manitoba, Canada; subspecies A.p. platyrhynchos, the Common Mallard.
BLUE-WINGED TEAL (Anas discors)
IDENTIFICATION: A grayish brown duck with a light blue upper-wing patch, tawny spotted underparts, and white, crescent-shaped facial stripes in males. DISTRIBUTION: Northern and central North America; winters in Central America and northern South America. HABITAT: Marshes, lakes, streams. STUDY AREA: Delta Waterfowl Research Station, Lake Manitoba, Canada.
{492}
Social Organization
Mallard Ducks and Blue-winged Teals are highly sociable birds, usually congregating in their own flocks of hundreds or (in Mallards) even thousands for most of the year. During the breeding season, they typically form monogamous pairs, although many variations exist. As in many other duck species, heterosexual pairs usually separate soon after incubation begins. Females then incubate the eggs and raise their families on their own.

A homosexual pair of male Mallard Ducks engaging in synchronized preening
Description
Behavioral Expression: Female Mallards sometimes mount and copulate with other females in the early fall, when ducks congregate in groups and begin to establish pair-bonds. Two females may engage in the PUMPING display, a prelude or invitation to mating in which the head is bobbed up and down so that the bill touches the water in a horizontal position. Following this, one female flattens her body on the water and extends her neck, allowing the other female to mount. While copulating, the mounting female may grab her partner's neck feathers in her bill or gently peck at her head. After dismounting, she performs a concluding display (also shown by females in heterosexual interactions) in which she dips her head in the water and then shakes the drops down her back while beating her wings. Homosexual mountings occasionally occur later in the season between heterosexually paired females and single females.
Homosexual pair-bonds also occur in both male and female Mallards. As in heterosexual pairs, the two partners keep close company, swimming together as well as resting, preening, and feeding in perfect synchrony. Same-sex partners also "defend" their mate from the approach of other Mallards. Females use a special INCITING display for this, in which they trail their partner while looking back over their shoulder and making a trembling call. Overt sexual activity is not generally a feature of same-sex partnerships, however: drake pairs, for example, engage in mutual head pumping and feather ruffling (which are preludes to copulation) but neither partner mounts the other or invites his mate to mount him. Interestingly, though, some males in homosexual pairs have been observed attempting to rape or forcibly copulate with males outside their pair-bond (just the way drakes in heterosexual pairs often participate in nonmonogamous raping of females — see below). Among females, homosexual pair-bonds are more ephemeral, generally occurring only in the pre-and postbreeding seasons. {493}
Some drake pairs are also temporary, while others are long-lasting, persisting for years and possibly even for life.
Male Mallards that have been raised together also frequently develop homosexual bonds of great strength and longevity. When large numbers of such birds are present, they often form their own groups, known as CLUBS. They flock together for hours or even days at a time, excitedly running about and swimming together while quacking continuously. Sometimes a female associates herself with a drake pair to form a bisexual trio; although one or both males may mate with her, their homosexual bond remains primary. Less commonly, females that have been raised together may also form a pair-bond, jointly incubating a nest and coparenting any ducklings that may result from promiscuous matings with males.
Blue-winged Teal drakes will court each other in the absence of females, even competing and fighting with one another for the attentions of another male.

Homosexual Mallard drakes tend to socialize primarily with
each other in their own flocks or "clubs"

Frequency: Homosexual copulations and pairings between female Mallard Ducks occur sporadically and are most common during the fall. In one study, roughly a quarter of the days on which sexual activity was observed included same-sex mountings. The proportion of male homosexual pairs varies between populations, anywhere from 2-19 percent of all pairs.
Orientation: Several forms of bisexuality occur among Mallards: females may participate in homosexual copulations while paired with a male, and both sexes may form seasonal homosexual attachments prior to or following a period of heterosexual mating. Some males are probably more exclusively homosexual, forming ongoing same-sex bonds that last for many years. In addition, most males probably have a bisexual potential: when raised in all-male groups, Mallards usually form {494}
lifelong homosexual pairs, and re-pair with other males on being "widowed." Nevertheless, even among males that have not been raised together, approximately 13-17 percent still participate in homosexual pairing for at least a portion of their lives. In Blue-winged Teals, homosexual behavior appears to be primarily a manifestation of a bisexual potential, since same-sex pairing or courtship have so far only been observed in males isolated from females.
Nonreproductive and Alternative Heterosexualities
Mallard pairs regularly engage in nonreproductive matings. For example, copulation is common during the five-to-seven months that heterosexual couples are together prior to the breeding season (when males are not producing sperm). Later in the breeding season, however, male-female relations are often marked by hostility, since forced copulation or rape is a common feature of opposite-sex interactions in both Mallards and Blue-winged Teals. Following egg-laying, male Mallards regularly abandon their female mates (who thus become "single parents"), congregate in all-male groups, and begin pursuing other females to try to forcibly mate with them. Rapes also occur between paired ducks in both species. As many as a 12-40 males may chase a single female in aerial or aquatic pursuit; drakes have even been known to grab and mount females underwater when they dive (attempting to escape), or to knock females to the ground in midflight. In some populations, as many as 7-10 percent of all females die each year as a result of drownings or other injuries incurred during rapes. Occasionally, males even try to mate with dead females. Even while they are still paired earlier in the breeding season, males frequently court and attempt to mate with (or rape) females other than their mate. About 3-7 percent of offspring are a result of such nonmonogamous matings, and in some populations multiple parentage occurs in at least 17-25 percent of all broods.
Mallards also sometimes form trio-bonds, either one male with two females (2-4 percent of all heterosexual bonds) or, more commonly, two males with one female (3-6 percent of all bonds). Paired males sometimes switch mates during the breeding season as well, and at least 9 percent of all heterosexual couples divorce between breeding seasons. Overall, long-term male-female pair-bonds (lasting two or more seasons) are rare in this species. Mallard mothers can be extremely aggressive in defense of their young, even killing other youngsters that stray from their own broods. In some populations the greatest cause of mortality among ducklings is attacks from other mothers. Occasionally, however, two broods join together and are defended by a single mother for short periods.
Other Species
Homosexual pairs also form among male Wood Ducks (Aix sponsa) that are raised together; such pairs are lifelong, and the two males may even search for nesting sites together each year. Female Chiloe Wigeons (Anas sibilatrix) have also been known to pair together in captivity; the partners remain bonded for many years and each lays eggs in their nest.
{495}
Sources
(* asterisked references discuss homosexuality/transgender)
Bailey, R. O., N. R. Seymour, and G. R. Stewart (1978) "Rape Behavior in Blue-winged Teal." Auk 95:188-90.
Barash, D. P. (1977) "Sociobiology of Rape in Mallards (Anas platyrhynchos): Responses of the Mated Male." Science 197:788-89.
Boos, J. D., T. D. Nudds, and K. Sjoberg (1989) "Posthatch Brood Amalgamation by Mallards." Wilson Bulletin 101:503-5.
* Bossema, I., and E. Roemers (1985) "Mating Strategy, Including Mate Choice, in Mallards." Ardea 73:147-57.
Cheng, K. M., J. T. Burns, and F. McKinney (1983) "Forced Copulation in Captive Mallards. III. Sperm Competition." Auk 100:302-10.
Evarts, S., and C. J. Williams (1987) "Multiple Paternity in a Wild Population of Mallards." Auk 104:597-602.
* Geh, G. (1987) "Schein-Kopula bei Weibchen der Stockente Anas platyrhynchos [Pseudo-Copulation of Female Mallard Ducks]." Anzeiger der Ornithologischen Gesellschaft in Bayern 26:131-32.
* Hochbaum, H. A. (1944) The Canvasback on a Prairie Marsh. Washington, D.C.: American Wildlife Institute.
Huxley, J. S. (1912) "A 'Disharmony' in the Reproductive Habits of the Wild Duck (Anas boschas L.)." Biologisches Centralblatt 32:621-23.
* Lebret, T. (1961) "The Pair Formation in the Annual Cycle of the Mallard, Anas platyrhynchos L." Ardea 49:97-157.
* Lorenz, K. (1991) Here Am I — Where Are You? The Behavior of the Greylag Goose. New York: Harcourt Brace Jovanovich.
* --- (1935) "Der Kumpan in der Umwelt des Vogels." Journal fur Ornithologie 83:10-213, 289-413. Reprinted as "Companions as Factors in the Bird's Environment." In K. Lorenz (1970), Studies in Animal and Human Behavior, vol. 1, pp. 101-258. Cambridge, Mass.: Harvard University Press.
Losito, M. P., and G. A. Baldassarre (1996) "Pair-bond Dissolution in Mallards." Auk 113:692-95.
McKinney, F., S. R. Derrickson, and P. Minneau (1983) "Forced Copulation in Waterfowl." Behavior 86:250-94.
Mjelstad, H., and M. Saetersdal (1990) "Reforming of Resident Mallard Pairs Anas platyrhynchos, Rule Rather Than Exception?" Wildfowl 41:150-51.
Raitasuo, K. (1964) "Social Behavior of the Mallard, Anas platyrhynchos, in the Course of the Annual Cycle." Papers on Game Research (Helsinki) 24:1-72.
* Ramsay, A. O. (1956) "Seasonal Patterns in the Epigamic Displays of Some Surface-Feeding Ducks." Wilson Bulletin 68:275-81.
* Schutz, F. (1965) "Homosexualitat und Pragung: Eine experimentelle Untersuchung an Enten [Homosexuality and Developmental Imprinting: An Experimental Investigation of Ducks]." Psychologische Forschung 28:439-63.
* Titman, R. D., and J. K. Lowther (1975) "The Breeding Behavior of a Crowded Population of Mallards." Canadian Journal of Zoology 53:1270-83.
Weston, M. (1988) "Unusual Behavior in Mallards." Vogeljaar 36:259.
Williams, D. M. (1983) "Mate Choice in the Mallard." In P. Bateson, ed., Mate Choice, pp. 33-50. Cambridge: Cambridge University Press.
{496}
 Other Ducks
Other Ducks
LESSER SCAUP DUCK (Aythya affinis)
IDENTIFICATION: A broad-billed duck with a purplish black head and breast and white underparts in males, and a dark head and brownish plumage in females. DISTRIBUTION: Northern and central North America; winters in southern United States and Mexico. HABITAT: Lakes, marshes, lagoons. STUDY AREAS: Lake Manitoba (Delta Marsh) and near Erickson, Manitoba; Cariboo region of British Columbia, Canada, including Watson and 150 Mile Lakes.
AUSTRALIAN SHELDUCK (Tadorna tadornoides)
IDENTIFICATION: Cinnamon breast, dark green head and back, and white collar; adult females have white eye and bill rings. DISTRIBUTION: Southern Australia, Tasmania. HABITAT: Marshes, lakes, lagoons. STUDY AREA: Rottnest Island, Western Australia.
MUSK DUCK (Biziura lobata)
IDENTIFICATION: A large, grayish duck with a prominent lobe hanging from the lower bill, and a spike-fan tail. DISTRIBUTION: Southern Australia, Tasmania. HABITAT: Swamps, lakes, other wetlands. STUDY AREA: Kangaroo Lake, Victoria, Australia.
Social Organization
Lesser Scaup Ducks are highly social, gathering into large waterborne flocks or "rafts" that may number in the tens of thousands. They form pair-bonds during the mating season, but males typically leave their mates following egg-laying (see below) and join large all-male groups. Australian Shelducks also form mated pairs during the breeding season (both parents care for the young) but otherwise associate in flocks. Musk Ducks are largely solitary except during the mating season; adult males are territorial, and they are probably polygamous or promiscuous (copulating with more than one female).
Description
Behavioral Expression: Male Lesser Scaup Ducks occasionally try to copulate with one another; drakes who participate in such homosexual activity are usually unpaired birds. While same-sex mounting does not occur among females, {497}
coparenting does. In this species, males usually abandon their female mates shortly after incubation of the eggs begins. Most females take care of their young entirely on their own as single parents, but sometimes two females join together and help each other raise their families. Accompanying their combined broods of 20 or more ducklings, the two females cooperate in all parental duties, including coordinated defense of the youngsters. If a predator or intruder approaches, one female distracts it by boldly approaching and feigning an injury, while her partner stealthily leads all of the ducklings away to safety. As their offspring get older, however, female coparents show less of this "distraction" behavior; at the approach of a predator, they may depart with one another and temporarily leave their ducklings behind to fend for themselves. Occasionally three females join forces and raise their combined broods — as many as 50 or more ducklings — as a parenting trio. Interestingly, duckling survival rates are not significantly different in families with one as opposed to two (or three) female parents.
Female Australian Shelducks often court one another and form homosexual pair-bonds. In December, when birds begin pairing, females display to each other with ritual preening movements and chases. These often develop into a full-fledged WATER-THRASHING DISPLAY, in which one female swims toward the other while making sideways pointing movements with her outstretched head and neck. She may also dive and resurface, then chase after the other female. Her partner is frequently captivated by this performance and responds by enthusiastically diving and chasing in return; the two females may, as a result, form a bond that lasts until the next pairing season. Females that engage in homosexual courtship and pairing are usually younger adult or juvenile birds.
Male Musk Ducks perform an extraordinary courtship display that attracts both males and females. The male arches his back and lifts his head up, engorging his large throat pouch; at the same time he fans and bends his tail forward over his back at an extreme angle — a feat made possible because of two extra vertebrae. This gives the bird an astounding, reptilian appearance. While in this posture, he kicks both feet backward or to the side, producing enormously loud splashes or jets of water. Multiple kicks of various types are given in series, and often the courting male rapidly back-paddles in between kicks. He also produces a wide variety of sounds during these PADDLE-KICK, PLONK-KICK, and WHISTLE-KICK displays, including ker-plonks (made by the kicking and splashing) combined with whirr or cuc-cuc vocalizations, and even a distinctive whistling sound. In addition, many displaying males emit a musky odor (hence the name of the bird), and their plumage {498}
is so oily that puddles of oil may form on the surface of the water around them. This display — perhaps one of the most dramatic of all birds — draws both males and females, who crowd around the courting male. Often, males appear to be more attracted than females, swimming much closer to the displaying male and sometimes even making physical contact by gently and repeatedly nudging their breast against his shoulder. The displaying male is in a trancelike state and rarely responds directly to any of the onlookers. Indeed, although homosexual copulation has not been observed as a part of these displays, heterosexual mating has hardly ever been seen during such courtship sessions either.

A male Musk Duck (left) attracted to another male performing a "whistle-kick" as part of an elaborate courtship display sequence
Frequency: Homosexual mounting probably occurs only occasionally in Lesser Scaup Ducks, but female coparenting is a regular feature of some populations. As many as a quarter to a third or more of all families have two mothers, although in other populations they are less frequent, comprising about 12 percent of families in some years. Same-sex courtship and pairing occur frequently among younger Australian Shelduck females, while male Musk Ducks are routinely attracted to displaying males.
Orientation: A variety of bisexual arrangements characterize these duck species. Some female Lesser Scaups (perhaps 30-40 percent of the population) have a seasonal alternation between same-sex and opposite-sex pairings: they begin the breeding season in heterosexual pairs, but end it in same-sex associations. Most Australian Shelduck females in homosexual pairs probably go on to form heterosexual bonds as adults. Musk Duck males display to both females and other males; some of the males they attract are also interested in females, but others are apparently only attracted to the courting male.
Nonreproductive and Alternative Heterosexualities
As described above, separation of heterosexual pairs with subsequent female single-parenting (or coparenting) is the usual pattern for Lesser Scaup families. Several other alternative parenting and pairing arrangements occur in these species. Occasionally a female Lesser Scaup associates with a mated pair and even lays eggs in their nest. Musk Ducks often lay their eggs in other birds' nests, where they are foster-parented by both their own and foreign species — including many other kinds of ducks (e.g., the blue-billed duck, Oxyura australis) as well as Dusky Moorhens. For their part, Lesser Scaups occasionally raise ducklings of other species of ducks, hatched from eggs that have been laid in their nests by, for example, redheads (Aythya americana). Australian Shelducks sometimes foster-parent chicks as well: about 5 percent of all broods contain "extra" ducklings from other families, and about 1 percent of all ducklings are adopted or "exchanged" between families. Lesser Scaup ducklings are occasionally abandoned by their mothers and may be adopted into other families. Abandonment of eggs is also prevalent: female Lesser Scaups may desert entire clutches, while egg DUMPING is common in Australian Shelducks. Many Shelduck pairs copulate but then lay or abandon the resulting eggs in caves, along the shore, or on islands, never incubating or hatching {499}
them. Most of these pairs — who may constitute close to half the population — have been unable to secure a breeding territory of their own. Many other birds are non-breeders as well: a large proportion of Lesser Scaups of both sexes, and Australian Shelduck females, are younger birds that are sexually mature but unpaired. In addition, it is thought that reproduction in younger male Musk Ducks may be suppressed by the presence of older males.
Although many Australian Shelducks form long-lasting heterosexual bonds, about 10 percent of breeding pairs divorce, and many more juvenile pairs separate. In Lesser Scaups, nonmonogamous copulations are common, accounting for more than half of all heterosexual activity. Many of these are rapes or forced copulations performed by paired males on females other than their mate; occasionally groups of up to eight males will pursue a female and try to mate with her. Only about 20 percent of such rapes involve penetration — the male Lesser Scaup, like most waterfowl (but unlike most other birds), does have a penis. More than a quarter of all such attempts are nonreproductive, occurring too early in the breeding season, during incubation, after breeding, or on nonbreeding females. In fact, the highest rates of attempted rape occur on females just before their molting period, when they are nonfertilizable. In Australian Shelducks, it is the females who vigorously pursue males, often courting already paired drakes in dramatic aerial chases. One or several females may try to maneuver in between a mated pair to separate the male, even grabbing at the tail feathers of his mate to force her to change direction. Females frequently suffer broken wings and may even be killed when they hit obstacles during such high-speed chases.
Other Species
Interspecies homosexual pairs involving several other kinds of ducks and geese have been observed in captive birds. A pair consisting of a female Common Shelduck (Tadorna tadorna) with a female Egyptian Goose (Alopochen aegyptiacus), for example, both laid eggs in a shared nest and jointly incubated them.
Sources
(* asterisked references discuss homosexuality/transgender)
* Afton, A. D. (1993) "Post-Hatch Brood Amalgamation in Lesser Scaup: Female Behavior and Return Rates, and Duckling Survival." Prairie Naturalist 25:227-35.
--- (1985) "Forced Copulation as a Reproductive Strategy of Male Lesser Scaup: A Field Test of Some Predictions." Behavior 92:146-67.
--- (1984) "Influence of Age and Time on Reproductive Performance of Female Lesser Scaup." Auk 101:255-65.
Attiwell, A. R., J. M. Bourne, and S. A. Parker (1981) "Possible Nest-Parasitism in the Australian Stiff-Tailed Ducks (Anatidae: Oxyurini)." Emu 81:41-42.
Bellrose, F. C. (1976) Ducks, Geese, and Swans of North America. Harrisburg, PA: Stackpole.
Fullagar, P. J., and M. Carbonell (1986) "The Display Postures of the Male Musk Duck." Wildfowl 37:142-50.
Gehrman, K. H. (1951) "An Ecological Study of the Lesser Scaup Duck (Aythya affinis Eyton) at West Medical Lake, Spokane County, Washington." Master's thesis, State College of Washington (Washington State University).
* Hochbaum, H. A. (1944) The Canvasback on a Prairie Marsh. Washington, D.C.: American Wildlife Institute.
* Johnsgard, P. A. (1966) "Behavior of the Australian Musk Duck and Blue-billed Duck." Auk 83:98-110.
* Low, G. C., and Marquess of Tavistock (1935) "The Extent to Which Captivity Modifies the Habits of Birds." Bulletin of the British Ornithologists' Club 55:144-54. {500}
* Lowe, V. T. (1966) "Notes on the Musk Duck Biziura lobata." Emu 65:279-89.
* Munro, J. A. (1941) "Studies of Waterfowl in British Columbia: Greater Scaup Duck, Lesser Scaup Duck." Canadian Journal of Research, section D 19:113-38.
O'Brien, R. M. (1990) "Musk Duck, Biziura lobata." In S. Marchant and P. J. Higgins, eds., Handbook of Australian, New Zealand, and Antarctic Birds, vol. 1, pp. 1152-60. Melbourne: Oxford University Press.
Oring, L. W. (1964) "Behavior and Ecology of Certain Ducks During the Postbreeding Period." Journal of Wildlife Management 28:223-33.
* Riggert, T. L. (1977) "The Biology of the Mountain Duck on Rottnest Island, Western Australia." Wildlife Monographs 52:1-67.
Rogers, D. I. (1990) "Australian Shelduck, Tadorna tadornoides." In S. Marchant and P. J. Higgins, eds., Handbook of Australian, New Zealand, and Antarctic Birds, vol. 1, pp. 1210-18. Melbourne: Oxford University Press.
{501}
OTHER AQUATIC BIRDS
 Auks and Albatrosses
Auks and Albatrosses
COMMON MURRE or GUILLEMOT (Uria aalge)
IDENTIFICATION: A gull-sized, web-footed bird with contrasting black upperparts and white underparts; some individuals have a white eye ring. DISTRIBUTION: Northern oceans and adjacent coasts. HABITAT: Marine coasts, bays, islands. STUDY AREAS: Gannet Islands, Labrador, Canada; Skomer Island, Wales; subspecies U.a. aalge and U.a. albionis.
LAYSAN ALBATROSS (Diomedea immutabilis)
IDENTIFICATION: A large, white-plumaged, gull-like bird with an enorous wingspan (over 6 1/2 feet), a dark back, and a grayish black wash on the face. DISTRIBUTION: Northern Pacific Ocean. HABITAT: Oceangoing; breeds on oceanic islands. STUDY AREA: Eastern Island in the Midway Atoll.
Social Organization
Common Murres and Laysan Albatrosses spend eight to nine months of the year at sea (often in large flocks for Murres). The remainder of the time, they gather at traditional nesting sites in extraordinary densities — Murre colonies, for example, can contain hundreds of thousands of pairs. The mating system is a combination of long-term pair-bonds and promiscuous copulations.
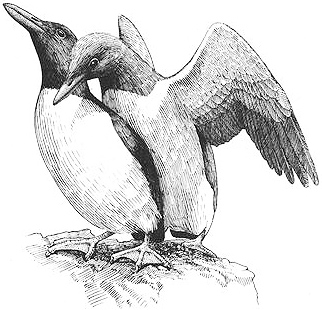
A male Common Murre attempting to forcibly copulate with another male
Description
Behavioral Expression: Male Common Murres — usually heterosexually paired — often try to copulate with birds other than their mate, including other {502}
males. Homosexual mountings — like heterosexual promiscuous mountings — are usually performed on birds returning to the colony after having been away (for example, while feeding). Immediately upon spotting an arriving male (or female), another male runs toward him, making a harsh, yodel-like crowing sound. He then hooks his neck around the other male and attempts to copulate with him. The other male usually prevents or resists the mounting attempt by standing upright, running away, or directly attacking him. Homosexual mountings also take place during "gang rape" attempts, which occur in 20-30 percent of all promiscuous matings. Groups of males — sometimes as many as ten at a time — gather to try to forcibly copulate with the same female, and occasionally males also mount each other during the ensuing sexual activity.
A similar form of rape occurs among Laysan Albatrosses. Early in the breeding season, males often leave their partner's side to try to copulate with males or females that are passing through the breeding colony. This is especially true if they momentarily and inadvertently spread and droop their wings (signals usually given by a female before copulation). Groups of five or six males often pursue the same individual, all jostling to mount him or her; typically a male will hook his bill across the neck of the bird being pursued, to throw it off balance. Homosexual mountings are common in these group rape attempts, and "pile-ups" or stacks of up to four males mounted on top of each other have been observed. Rape attempts — whether on males or females — never result in ejaculation, since the bird being mounted always resists the advances of the pursuing bird. A completely different homosexual activity also occurs in this species: occasionally two birds of the same sex perform an elaborate courtship dance with one another. This complex synchronized display involves more than 25 different postures. The two birds stand facing each other, stretching their heads upward during SKY CALLS and SKY MOOS, clap their bills, and bow, strut, and circle around their partner, all the while making a cacophony of clicking, whinnying, wailing, and grunting sounds.
Frequency: At least 5-6 percent of all promiscuous mating attempts on arriving Common Murres are homosexual, and one out of ten arriving males is mounted by another male (compared to three out of four arriving females). Homosexual copulation attempts probably represent 1 percent or less of all mountings (both {503}
promiscuous and between pair-bonded birds). In Laysan Albatrosses, rape attempts are frequent before egg laying and probably occur with equal regularity on males and females. Approximately 9 percent of courtship dances take place between two females and 4 percent between two males.
Orientation: About two-thirds of all male Common Murres participate in promiscuous copulations; only a fraction of these engage in homosexual mountings. Male Laysan Albatrosses are as likely to pursue and mount other males as females in their rape attempts. Although it is difficult to draw firm conclusions without detailed study of individual birds, most males that engage in homosexual behavior in these species are probably functionally bisexual, since they are usually already paired with a female (although a few Common Murres who participate in such activity may be unpaired). However, their primary orientation is probably heterosexual since relatively few of their sexual interactions are with other males. The same probably holds for males being mounted by other males: because they usually resist forced mounts by other males, it is likely that most such males are heterosexually oriented. However, most females also resist forced mountings by males, so it is possible that males are reacting negatively to the forced nature of the copulation attempt, as much if not more so than to the sex of the bird mounting them.
Nonreproductive and Alternative Heterosexualities
As mentioned above, promiscuous copulations occur frequently in these species. About 10 percent of all Common Murre matings are forced copulations between a male and a female other than his partner, and on some days each female is subjected to such a rape attempt nearly every hour. Females usually respond aggressively to such attacks, and their mates also try to defend them, although sometimes an intruding male will actually disrupt a copulation between a mated pair by knocking the male off his partner's back. In about 15 percent of all promiscuous matings, the female does not react aggressively and appears to cooperate in allowing the male to make genital contact. Female Laysan Albatrosses always resist rape attempts and may be severely injured in the process: one female was attacked by four different gangs of males in ten minutes, losing an eye and sustaining severe wing injuries. However, forced copulations in this species are always nonprocreative since sperm is never transferred. Many promiscuous matings in Common Murres are nonreproductive as well: cloacal contact often does not occur (less than 1 in 200 such matings result in insemination), and during group promiscuous matings, males often mount on any part of the female's body, including her head. In addition, about 15-30 percent of promiscuous copulations occur outside of the female's fertile period. The same is also true for sexual activity between mated partners: copulation begins as long as four to five months before the start of egg laying, and half of all heterosexual matings in some populations occur during nonfertilizable periods. In addition, almost a quarter of pair copulations do not involve genital contact. In Common Murres — as in most other birds — females have the remarkable ability to store sperm in special ducts in their reproductive tract, allowing {504}
them to inseminate their eggs even when not directly engaging in reproductive copulations.
Other forms of nonprocreative sexuality also occur. Nonbreeding female Common Murres often solicit promiscuous matings from males, while nonbreeding pairs or those who have lost their young (which can make up as much as a third of all pairs) frequently continue to copulate throughout the season. Nonbreeding Laysan Albatross pairs also sometimes engage in copulation. Birds in this species do not reproduce until they are 6-16 years old, even though they mature at one year old and may form pairs fully two years before they actually breed. Similarly, younger Common Murres usually delay breeding until they are five years old, congregating in CLUBS on the tidal rocks beneath the breeding colonies. Such nonbreeders make up approximately 13 percent of the population; among adults, 5-10 percent of birds do not breed each year, and more than a third skip breeding for at least one season during their life. In addition, masturbatory activity — birds mount and "copulate" with clumps of grass — was recently discovered in a closely related species, the thick-billed murre (Uria lomvia); it is likely that similar behavior also occurs in Common Murres.
A variety of alternative parenting arrangements are also found in these species. About 8 percent of all Common Murre chicks have "baby-sitters" — a pair of birds other than their parents who help brood (keep warm), protect, and sometimes feed the chick (even when the youngster's parents are not away). Most such helpers are nonbreeders; others have tried but failed to breed, while some have finished raising their family or are also taking care of their own chick. In addition, pair separation and single parenting is routine in Common Murres: when a chick is old enough to leave the colony, only its father accompanies it to sea, feeding and chaperoning it for up to 12 weeks without his female partner. In Laysan Albatrosses, heterosexual parents are together at the nest for a remarkably short time — only 5-10 days out of the 230-day breeding season. Eggs are sometimes temporarily "adopted" by other birds who incubate them when the parents are away from the nest. Nonbreeding females have even been known to "join" existing pairs and regularly take turns with the parents incubating their egg. Sometimes females also lay a second egg in a stranger's nest. Reproduction in this species is often fraught with difficulties, however. More than 20 percent of parents (both males and females) desert their nests — often when their partner fails to return for an incubation shift on time — and couples also occasionally divorce (2 percent of all pairs). Once the chicks have hatched, they are often subjected to abuse from neighboring birds, who may savagely peck, stab, bite, and occasionally even kill the youngsters if they stray too close.
Other Species
Homosexual copulations are common in another species of auk, the Razorbill (Alca torda), where 41 percent of nonmonogamous mountings (about 18 percent of all mountings) are between males. Up to 200 or more such mountings have been observed each season in some populations. Nearly two-thirds of all males mount other males (an average of 5 partners, sometimes as many as 16) and more than 90 percent of males receive mounts from other males. Older males participate more {505}
often than younger ones, and mountings are occasionally reciprocal. Like females, males usually resist such promiscuous mating attempts: although the mounter usually tries to achieve cloacal (genital) contact, only about 1 percent of same-sex mountings include genital contact or ejaculation (compared to 12 percent of promiscuous heterosexual mounts).
Sources
(* asterisked references discuss homosexuality/transgender)
Birkhead, T. R. (1993) Great Auk Islands. London: T. and A.D. Poyser.
* --- (1978a) "Behavioral Adaptations to High Density Nesting in the Common Guillemot Uria aalge." Animal Behavior 26:321-31.
--- (1978b) "Attendance Patterns of Guillemots Uria aalge at Breeding Colonies on Skomer Island." Ibis 120:219-29.
Birkhead, T. R., and P. J. Hudson (1977) "Population Parameters for the Common Guillemot Uria aalge." Ornis Scandinavica 8:145-54.
Birkhead, T. R., S. D. Johnson, and D. N. Nettleship (1985) "Extra-pair Matings and Mate Guarding in the Common Murre Uria aalge." Animal Behavior 33:608-19.
Birkhead, T. R., and D. N. Nettleship (1984) "Alloparental Care in the Common Murre (Uria aalge)." Canadian Journal of Zoology 62:2121-24.
Fisher, H. I. (1975) "The Relationship Between Deferred Breeding and Mortality in the Laysan Albatross." Auk 92:433-41.
* --- (1971) "The Laysan Albatross: Its Incubation, Hatching, and Associated Behaviors." Living Bird 10:19-78.
--- (1968) "The 'Two-Egg Clutch' in the Laysan Albatross." Auk 85:134-36.
Fisher, H. I., and M. L. Fisher (1969) "The Visits of Laysan Albatrosses to the Breeding Colony." Micronesica 5:173-221.
Fisher, M. L. (1970) The Albatross of Midway Island: A Natural History of the Laysan Albatross. Carbondale, Ill.: Southern Illinois University Press.
* Frings, H., and M. Frings (1961) "Some Biometric Studies on the Albatrosses of Midway Atoll." Condor 63:304-12.
Gaston, T., and K. Kampp (1994) "Thick-billed Murre Masturbating on Grass Clump." Pacific Seabirds 21:30.
Harris, M. P., and S. Wanless (1995) "Survival and Non-Breeding of Adult Common Guillemots Uria aalge." Ibis 137:192-97.
* Hatchwell, B. J. (1988) "Intraspecific Variation in Extra-pair Copulation and Mate Defence in Common Guillemots Uria aalge." Behavior 107:157-85.
Hudson, P. J. (1985) "Population Parameters for the Atlantic Alcidae." In D. N. Nettleship and T. R. Birkhead, eds., The Atlantic Alcidae, pp. 233-61. London: Academic Press.
Johnson, R. A. (1941) "Nesting Behavior of the Atlantic Murre." Auk 58:153-63.
Meseth, E. H. (1975) "The Dance of the Laysan Albatross, Diomedea immutabilis." Behavior 54:217-57.
Rice, D. W., and K. W. Kenyon (1962) "Breeding Cycles and Behavior of Laysan and Black-footed Albatrosses." Auk 79:517-67.
Tuck, L. M. (1960) The Murres: Their Distribution, Populations, and Biology. Ottawa: Canadian Wildlife Service.
* Wagner, R. H. (1996) "Male-Male Mountings by a Sexually Monomorphic Bird: Mistaken Identity or Fighting Tactic?" Journal of Avian Biology 27:209-14.
--- (1991) "Evidence That Female Razorbills Control Extra-Pair Copulations." Behavior 118:157-69.
{506}
 Cormorants
Cormorants
GREAT CORMORANT (Phalacrocorax carbo)
IDENTIFICATION: A large (3 foot), black, web-footed bird with a white throat and white filamentary plumes on the nape. DISTRIBUTION: Throughout Europe, Australasia, Africa, and Atlantic North America. HABITAT: Seacoasts, lakes, rivers. STUDY AREAS: Shinobazu Pond, Tokyo, Japan; Amsterdam Zoo, the Netherlands; subspecies P.c. sinensis and P.c. hanedae.
EUROPEAN SHAG (Phalacrocorax aristotelis)
IDENTIFICATION: Similar to Great Cormorant, but smaller and uniformly black, with a prominent forehead crest. DISTRIBUTION: Northwestern Europe, Mediterranean basin. HABITAT: Coastal waters; nests on cliffs. STUDY AREA: Lundy Island in the Bristol Channel, England; subspecies
P.a. aristotelis.
Social Organization
Great Cormorants and Shags form mated pairs and generally nest in colonies, which may contain as many as 20,000 pairs in some populations of Great Cormorants. Outside of the mating season, these species are moderately gregarious, wandering solitarily but sometimes forming flocks.
Description
Behavioral Expression: Homosexual pairs consisting of two males sometimes form in Great Cormorants and last for up to five years (heterosexual pairs in this species are usually seasonal but may also last for several years). Male pairs often build oversize nests because both birds contribute to the construction of the nest. They often sit on the nest as if incubating eggs; similar behavior is also seen in heterosexual pairs prior to egg laying. In some homosexual pairs, one partner may use vocalizations that are typical of females (such as panting or purring sounds), or else calls that are intermediate between male and female vocal patterns. Male pairs are sometimes incestuous, composed of two brothers.
In European Shags, males occasionally court other males. As one male approaches — hopping along the rocks, pausing every now and then in an erect pose known as the UPRIGHT-AWARE POSTURE — the other male performs two displays. In {507}
the DART-GAPE, he pulls his head back and then darts it forward, at the same time opening his bill to expose the yellow interior and fanning his tail. In the THROW-BACK, he arches his neck along his back and points his beak upward while quivering his throat pouch. Sometimes the courting male will become aggressive and attack another male that approaches too closely, which also happens frequently when females approach courting males.
Frequency: Homosexual pairs and courtship occur only occasionally in these two species: no more than perhaps 1 in 500 pairs of Great Cormorants, for example, is composed of two males.
Orientation: Great Cormorants in homosexual pairs are sometimes sequentially bisexual, divorcing their male partners and going on to breed in heterosexual pairs. However, others re-pair with another male partner, and some homosexual pairs appear to last much longer, perhaps even for the birds' lifetimes — in which case such individuals are exclusively (or extensively) homosexual for a significant part of their lives. Male European Shags that court other males are simultaneously bisexual, alternating heterosexual courtship with homosexual interactions (and probably a greater proportion of the former).
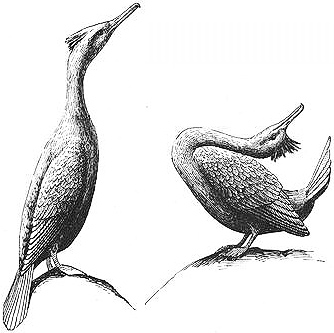
Courtship between two male European Shags: the "uprightaware" posture (left) and the "throw-back" display
Nonreproductive and Alternative Heterosexualities
Several forms of nonprocreative sexual behavior are exhibited by these Cormorants. REVERSE mountings constitute 8 percent of European Shag heterosexual copulations (and also occur in Great Cormorants), while at least a quarter of all sexual activity takes place prior to the female's fertilizable period. Great Cormorants sometimes copulate during the incubation period, while heterosexual copulations may continue even after Shag chicks have hatched. In a few cases, adult male Shags have been observed mating with their own chicks, and incestuous pairings sometimes also develop between brothers and sisters when they are still young. {508}
Nearly half of all heterosexual copulations in Shags involve mounting without genital contact, often because the female will not permit it. In addition, males are frequently hostile to females during the early phases of courtship (as noted above).
Nonmonogamous matings and courting of birds other than one's partner occur in both of these species. In European Shags, for example, 14 percent of all copulations are promiscuous. Almost 18 percent of all chicks are fathered by a male other than their mother's mate, but nearly 80 percent of all nonmonogamous matings are nonreproductive, taking place before females can be fertilized. At least 4 percent of all chicks are related to neither of the parents caring for them; this results from adoption and from females' laying eggs in nests other than their own. About 3-5 percent of male Shags bond polygamously with two females; in addition, 30 — 40 percent of heterosexual Shag pairs divorce and re-pair with new mates the next season. Individuals may also change mates during the season. Some Shag parents are severely neglectful, refusing to feed their offspring, who may, as a result, die of starvation. In addition, about a third of all eggs lost through breakage result from interference by the females in polygamous associations. Finally, nonbreeding is a regular feature of some Shag populations: on average, 12-25 percent of all adults skip breeding at least once during their lifetime, and in some years as many as 60 percent of all birds forgo reproduction.
Sources
(* asterisked references discuss homosexuality/transgender)
Aebischer, N. J., G. R. Potts, and J. C. Coulson (1995) "Site and Mate Fidelity of Shags Phalacrocorax aristotelis at Two British Colonies." Ibis 137:19-28.
Aebischer, N. J., and S. Wanless (1992) "Relationships Between Colony Size, Adult Non-Breeding, and Environmental Conditions for Shags Phalacrocorax aristotelis on the Isle of May, Scotland." Bird Study 39:43-52.
* Fukuda, M. (1992) "Male-Male Pairing of the Great Cormorant (Phalacrocorax carbo hanedae)." Colonial Waterbird Society Bulletin 16:62-63.
Graves, J., R. T. Hay, M. Scallan, and S. Rowe (1992) "Extra-Pair Paternity in the Shag, Phalacrocorax aristotelis as Determined by DNA Fingerprinting." Journal of Zoology, London 226:399-408.
Harris, M. P. (1982) "Promiscuity in the Shag as Shown by Time-Lapse Photography." Bird Study 29:149-54.
Johnsgard, P. A. (1993) Cormorants, Darters, and Pelicans of the World. Washington and London: Smithsonian Institution Press.
* Kortlandt, A. (1995) "Patterns of Pair-Formation and Nest-Building in the European Cormorant, Phalacrocorax carbo sinensis." Ardea 83:11-25.
* --- (1949) "Textuur en structuur van het broedvoorbereidingsgedrag bij de aalscholver [Texture and Structure of Brooding-Preparatory Behavior in the Cormorant]." Ph.D. thesis, University of Amsterdam.
* Snow, B. K. (1963) "The Behavior of the Shag." British Birds 56:77-103, 164-86.
{509}
 Grebes
Grebes
SILVERY GREBE (Podiceps occipitalis)
IDENTIFICATION: A ducklike bird with grayish white plumage, bright red eyes, and yellow facial tufts. DISTRIBUTION: Western and southern South America. HABITAT: Lakes, marshy ponds. STUDY AREA: Laguna Nevada, southern Patagonia, Argentina.
HOARY-HEADED GREBE (Poliocephalus poliocephalus)
IDENTIFICATION: Similar to Silvery Grebe, but with a buff or chestnut wash on the breast, white streaks on the head, and black-and-white eyes. DISTRIBUTION: Australia, Tasmania. HABITAT: Wetlands, estuaries, bays. STUDY AREA: Lake Bathurst, New South Wales, Australia.
Social Organization
Both of these species of Grebes commonly socialize in pairs or small groups, and sometimes in dense flocks (which may contain several thousand birds in Hoary-headed Grebes). They nest in large colonies — up to 400 nests for Hoary-headed Grebes — and most form monogamous pair-bonds.
The "rearing" display of a Hoary-headed Grebe, used during courtships with same- and opposite-sex partners
Description
Behavioral Expression: Male Silvery Grebes occasionally mount other males, using the same position as for heterosexual copulation — although unlike in heterosexual mating, the mounted bird does not typically invite the other male to mount him. In Hoary-headed Grebes, two birds of the same sex sometimes perform courtship displays to each other on floating platforms, constructed of water vegetation, and perhaps also mount one another. One such display is called REARING, in which {510}
the bird lifts its body up, ruffles its mantle feathers, and extends its head and neck downward. This may lead to one bird INVITING the other to mount, by settling back down, kinking its neck, and resting its throat on the nest.
Frequency: Same-sex activity occurs only occasionally in these two species of Grebes. In Silvery Grebes, for example, about 1 in 300 mounts is between two males.
Orientation: Grebes that participate in same-sex activities are probably bisexual, also courting and mating with members of the opposite sex.
Nonreproductive and Alternative Heterosexualities
In these two species of Grebes — as in many other Grebes — REVERSE heterosexual mountings are common. An average of 27 percent of all copulations among Silvery Grebes involve females mounting males, and early in the breeding season as many as 40 percent of all mounts are reverse. Their prevalence during this period — long before fertilization is possible — indicates that this form of behavior is decidedly nonprocreative. In addition, ejaculation does not usually occur during reverse mounts, although females typically perform tail thrusting, perhaps to facilitate genital contact. Grebes also copulate repeatedly, with as many as five or six matings taking place over 15-20 minutes; during this activity, males and females may also alternate positions in a form of reciprocal mounting. Although most heterosexual mating in Hoary-headed Grebes takes place between pair members, some copulations also occur between unpaired birds.
Sources
(* asterisked references discuss homosexuality/transgender)
* Fjeldsa, J. (1983) "Social Behavior and Displays of the Hoary-headed Grebe Poliocephalus poliocephalus." Emu 83:129-40.
Fjeldsa, J. and N. Krabbe (1990) Birds of the High Andes. Copenhagen: Zoological Museum, University of Copenhagen; Svendborg, Denmark: Apollo Books.
Johnsgard, P. A. (1987) Diving Birds of North America. Lincoln and London: University of Nebraska Press.
* Nuechterlein, G. L., and R. W. Storer (1989) "Reverse Mounting in Grebes." Condor 91:341-46.
* O'Brien, R. M. (1990) "Hoary-headed Grebe, Poliocephalus poliocephalus.' In S. Marchant and P. J. Higgins, eds., Handbook of Australian, New Zealand, and Antarctic Birds, vol. 1, pp. 100-107. Melbourne: Oxford University Press.
Storer, R. W. (1969) "The Behavior of the Horned Grebe in Spring." Condor 71:180-205.
{511}
WADING BIRDS
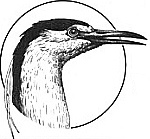 Herons
Herons
BLACK-CROWNED NIGHT HERON (Nycticorax nycticorax)
IDENTIFICATION: A stocky, medium-sized (2 foot long) heron with a black crown and back, white underparts, gray wings, and white ribbon plumes at the nape of the neck. DISTRIBUTION: Over much of Europe, Asia, Africa, and North and South America. HABITAT: Wetlands. STUDY AREAS: American Museum of Natural History, New York; Altenberg, Austria; subspecies N.n. hoactli and
N.n. nycticorax.
Social Organization
Black-crowned Night Herons are fairly gregarious birds, gathering in colonies that may contain hundreds or thousands of individuals nesting close together. Monogamous pairs predominate during the mating season.
Description
Behavioral Expression: Male Black-crowned Night Herons sometimes court other males and form homosexual pairs. To attract other birds, a male performs the SNAP-HISS CEREMONY, in which he repeatedly extends and lowers his head with erected plumes while "treading" with his feet and making a combined snapping (or clicking) and hissing sound. This courtship display is directed at both males and females. While most males are not interested in the ceremony, those that are attracted go on to participate in an OVERTURE display (also used in heterosexual courtship). In this activity, one or both males stretch their heads forward (again with feathers raised) while making their eyeballs protrude from their sockets, at the same time clicking and touching their bills. Males in homosexual pairs also mount each other (in the position used for heterosexual copulation); both males may mount or be {512}
mounted, although individuals sometimes show a preference for one or the other activity. Typically one male in the pair also builds a nest out of twigs; sometimes this occurs before the pair-bond forms, or else the two males may search for a nest site together. Once the pair-bond is established, both males vigorously defend their territory against other birds. Homosexual pair-bonds are strong, lasting the entire breeding season (as do heterosexual bonds). Young Black-crowned Night Herons also form pairs or "companionships," some of which are between birds of the same sex (although no overt sexual behavior occurs between companions of either the same or opposite sex). Occasionally, a juvenile female may even bond with more than one other female. Although no homosexual pairs between adult females have yet been observed, females sometimes perform typically male courtship displays such as the snap-hiss ceremony.
Frequency: In some captive populations, homosexual pairs in adult males make up more than 20 percent of all pairings, while 38 percent of juvenile pairs are same-sex (three-quarters of which are between females). The incidence of same-sex pairs in the wild is not known. Adult male partners may mount each other more than 30 times over the mating season.
Orientation: During the courtship phase, males exhibit a form of simultaneous bisexuality by displaying to both sexes, and some males are clearly more attracted to same-sex courtships than others. Most young females that form same-sex bonds only pair with other females, while a few form both homosexual and heterosexual pair-bonds. Once a homosexual bond has formed, though, the birds will maintain the bond even if they are separated for several weeks, returning to their same-sex partner when reunited rather than establishing a heterosexual bond. Some birds may exhibit sequential bisexuality, forming heterosexual bonds after participating in homosexual pairings when young (or vice versa).
Nonreproductive and Alternative Heterosexualities
Male-female relations in Black-crowned Night Herons are sometimes fraught with complications. Heterosexual copulation is often incomplete because females refuse to cooperate in mating. In addition, in the early stages of courtship, males are often aggressive toward any bird that approaches them, including females. Chicks that hatch late are usually deserted by their parents after their siblings have fledged; they often move to other nests and are adopted by those families. Adults also occasionally accept eggs laid in their nest by other herons such as great egrets (Casmerodius albus) or lay their own eggs in nests of other species such as snowy egrets (Egretta thula).
Sources
(* asterisked references discuss homosexuality/transgender)
Allen, R. P., and F. P. Mangels (1940) "Studies of the Nesting Behavior of the Black-crowned Night Heron." Proceedings of the Linnaean Society of New York 50-51:1-28.
Cannell, P. E., and B. A. Harrington (1984) "Interspecific Egg Dumping by a Great Egret and Black-crowned Night Herons." Auk 101:889-91. {513}
Davis, W. E., Jr. (1993) "Black-crowned Night Heron (Nycticorax nycticorax)." In A. Poole and F. Gill, eds., The Birds of North America: Life Histories for the 21 st Century, no. 74. Philadelphia: Academy of Natural Sciences; Washington, D.C.: American Ornithologists' Union.
Gross, A. O. (1923) "The Black-crowned Night Heron (Nycticorax nycticorax naevius) of Sandy Neck." Auk 40:1-30, 191-214.
Kazantzidis, S., V. Goutner, M. Pyrovetsi, and A. Sinis (1997) "Comparative Nest Site Selection and Breeding Success in 2 Sympatric Ardeids, Black-Crowned Night-Heron (Nycticorax nycticorax) and Little Egret (Egretta garzetta) in the Axios Delta, Macedonia, Greece." Colonial Waterbirds 20:505-17.
* Lorenz, K. (1938) "A Contribution to the Comparative Sociology of Colonial-Nesting Birds." In F. C. R. Jourdain, ed., Proceedings of the Eighth International Ornithological Congress, Oxford (July 1934), pp. 207-21. Oxford: Oxford University Press.
McClure, H. E., M. Yoshii, Y. Okada, and W. F. Scherer (1959) "A Method for Determining Age of Nestling Herons in Japan." Condor 61:30-37.
* Noble, G. K., and M. Wurm (1942) "Further Analysis of the Social Behavior of the Black-crowned Night Heron." Auk 59:205-24.
* Noble, G. K., M. Wurm, and A. Schmidt (1938) "Social Behavior of the Black-crowned Night Heron." Auk 55:7-40.
Schorger, A. W. S. (1962) "Black-crowned Night Heron." In R. S. Palmer, ed., Handbook of North American Birds, vol. 1: Loons through Flamingos, pp. 472-84. New Haven and London: Yale University Press.
{514}
 Herons and Egrets
Herons and Egrets
CATTLE EGRET (Bubulcus ibis)
LITTLE EGRET (Egretta garzetta)
IDENTIFICATION: Long-legged, typically white herons with ornamental, filamentous plumes on the back, breast, and nape; these are golden-buff-colored in the Cattle Egret. DISTRIBUTION: Throughout Africa, southern Europe, Australasia, and (in Cattle Egret) North and South America. HABITAT: Variable, including swamps, marshes, rivers, lakes, meadows. STUDY AREA: Near Tsu City, Japan; subspecies Bj. coromanda and E.g. garzetta.
LITTLE BLUE HERON (Egretta caerulea)
IDENTIFICATION: Similar to Little Egret but with slaty-gray plumage and a reddish brown head and neck. DISTRIBUTION: Southeastern United States to northern South America. HABITAT: Lakes, marshes, streams. STUDY AREA: Swan Lake, Arkansas; Cliftonville, Massachusetts.
GRAY HERON (Ardea cinerea)
IDENTIFICATION: A large (3 foot long) heron with a gray back, white head and neck, and black "eyebrow" stripe and nape plumes. DISTRIBUTION: Throughout Eurasia and Africa. HABITAT: Wetlands. STUDY AREA: Donana National Park, Spain; subspecies
A.c. cinerea.
Social Organization
Herons and Egrets are highly social birds, nesting in dense colonies that may include birds of several different species. During the mating season the primary social unit is the monogamous pair, although several alternative mating systems occur (see below). Outside of the breeding season, they may be found either singly or in flocks.
Description
Behavioral Expression: In all four of these Heron and Egret species, males that are paired to females often copulate with birds other than their mates; in some cases, these involve homosexual copulations with other males who are themselves {515}
also paired to females. Homosexual mountings always take place during the mating season. In Little Egrets, mountings between males are most common during the early stages of heterosexual pair formation (before nest-building begins), while in Little Blue Herons at least some homosexual activity takes place during the incubation period, since males have been seen mounting other males that are sitting on eggs. Typically, males mount birds in neighboring nests, although in Little Egrets and Little Blue Herons males may travel to other areas of the breeding colony to engage in "extramarital" or promiscuous copulations (both homosexual and heterosexual).
In Cattle Egrets (and probably the other species as well), homosexual mountings always take place on the mountee's nest. In a typical encounter, the male seeking an "extramarital" liaison approaches another male, uttering RICK RACK calls (a harsh double croaking sound, also used in heterosexual encounters). The first male then mounts the other bird and crouches on his back; some males only act as mounters in homosexual copulations, others only as mountees, while some males perform both roles. In Little Blue Herons and Cattle Egrets, homosexual mountings may also occur when one male mounts another male who is himself attempting to copulate with a female; sometimes, "pile-ups" of three or four males on top of each other may develop in this way. Usually the mountee is aggressive toward the male mounting him and does not permit cloacal contact. Similarly, male-female "extramarital" copulations are rarely completed, owing to resistance by the female or defense by her mate. In Cattle Egrets, nearly a quarter of all such heterosexual mounting attempts do not involve cloacal contact, while in Little Egrets more than 85 percent of such opposite-sex copulations are "incomplete."
Frequency: Homosexual mountings can be quite common: in Little Egrets, for example, more than 100 mounts between males were recorded over four months in one colony, with such copulations comprising 5-6 percent of all "extramarital" sexual activity. In Little Blue Herons, homosexual mountings make up 3-6 percent of all copulations outside the pair-bond. Mounts between males represent 5 percent of "extramarital" copulations and 3 percent of all copulations in Cattle Egrets, while in Gray Herons they constitute 8 percent of all promiscuous mountings and 1 percent of the total number of copulations. In 18 percent of "extramarital" copulation attempts on female Cattle Egrets, additional males mount other males in a pile-up.
Orientation: Since males that participate in homosexual activity almost always have female mates, they are technically bisexual (and some birds may even participate in "group" sexual activity involving both males and females simultaneously, as in Little Blue Herons). In Little Egrets, about one-quarter of the male population engages in homosexual mounting, in Gray Herons 5-7 percent of males are involved in such activity, while in Cattle Egrets six out of ten males in one colony participated in same-sex mounting. Some individuals seem to show more of a "predilection" for homosexual behavior than others. In Cattle Egrets, for example, certain males engage in "extramarital" mountings only with males rather than females, while in both this species and in Little Egrets, some individuals participate in {516}
same-sex activity noticeably more often than others. In addition, homosexual activity comprises a greater proportion of "extramarital" sexual activity for some males than for others.
Nonreproductive and Alternative Heterosexualities
As described above, "extramarital" or promiscuous heterosexual copulations occur commonly in all four of these species. In Cattle Egrets, as many as 60 percent of all mountings are by males on females other than their mates, while in Gray Herons such matings account for more than 12 percent of all sexual activity. Nearly a third of all Little Egret copulations are promiscuous. In fact, many copulations in this context are actually rapes, since the female is not a willing participant (although in both Cattle and Little Egrets, females may also consent to such matings). Up to 7 percent of Cattle Egret eggs may be fertilized by a male other than the bird's (social) father; however, many "extramarital" copulations are nonprocreative, since almost a quarter of all such matings take place when the female is already incubating her eggs. In addition to stepparenting of birds fathered by other males, several other alternative family arrangements occur: Cattle Egret trios of two females and one male may raise a family, while foster-parenting sometimes occurs when females lay their eggs in nests of other birds, including other species of Egrets and Herons.
Several mating behaviors in these species indicate that not all aspects of heterosexuality revolve around breeding. Cattle Egrets sometimes mate when fertilization is not possible, for example during incubation or chick-raising. And up to 14 percent of copulations between pair members may be "incomplete" in the sense that no genital contact or sperm transfer occurs — sometimes because the male is apparently not "interested" in mating even though his female partner is. In Little Blue Herons, some males copulate with females and yet remain "single" (i.e., do not pair-bond with them), while other males never pair with a female during the entire mating season. REVERSE mounts (females mounting males) also occur in Cattle Egrets, and in polygamous trios this sometimes results in a "pile-up" of three birds (one female mounting the second female who is mounting the male).
A number of violent and counterreproductive behaviors can make life harsh for young Egrets and Herons. In Little Blue Herons, infidelity often leads to abandonment of the nest by one or both partners (in part because eggs may be broken during the promiscuous sexual activity). Following a partner's injury, male Cattle Egrets have been known to destroy their own eggs and desert their mates for a new female. Male Gray Herons also occasionally destroy their eggs by stabbing at them. Nest and mate desertion (especially by females) are common in Little Egrets as well. Often the remaining bird will successfully raise the chicks as a single parent; sometimes, though, the chicks die as a result of desertion. If a single father pairs with a new female, she may kill his nestlings by pecking them to death, so that she can mate with him and raise her own offspring. Cannibalism by siblings or parents sometimes occurs in Gray Herons. In addition, Heron and Egret families are often systematically "pared down" because the youngest nestlings starve to death when they are unable to compete for food; more than three-quarters of all nestling deaths in Little Blue Herons are the result of such "brood reduction."
{517}
Sources
(* asterisked references discuss homosexuality/transgender)
Blaker, D. (1969) "Behavior of the Cattle Egret Ardeola ibis." Ostrich 40:75-129.
* Fujioka, M. (1996) Personal communication.
--- (1989) "Mate and Nestling Desertion in Colonial Little Egrets." Auk 106:292-302.
* --- (1988) "Extrapair Copulations in Little Egrets (Egretta garzetta)." Paper presented at the annual meeting of the Animal Behavior Society, University of Montana.
--- (1986a) "Infanticide by a Male Parent and by a New Female Mate in Colonial Egrets." Auk 103:619-21.
--- (1986b) "Two Cases of Bigyny in the Cattle Egret Bubulcus ibis." Ibis 128:419-22.
--- (1985) "Sibling Competition and Siblicide in Asynchronously-Hatching Broods of the Cattle Egret Bubulcus ibis." Animal Behavior 33:1228-42.
* Fujioka, M., and S. Yamagishi (1981) "Extramarital and Pair Copulations in the Cattle Egret." Auk 98:134-44.
Lancaster, D. A. (1970) "Breeding Behavior of the Cattle Egret in Colombia." Living Bird 9:167-94.
McKilligan, N. G. (1990) "Promiscuity in the Cattle Egret (Bubulcus ibis) Auk 107:334-41.
* Meanley, B. (1955) "A Nesting Study of the Little Blue Heron in Eastern Arkansas." Wilson Bulletin 67:84-99.
Milstein, P. le S., I. Prestt, and A. A. Bell (1970) "The Breeding Cycle of the Gray Heron." Ardea 58:171-257.
* Ramo, C. (1993) "Extra-Pair Copulations of Gray Herons Nesting at High Densities." Ardea 81:115-20.
Rodgers, J. A., Jr. (1980a) "Little Blue Heron Breeding Behavior." Auk 97:371-84.
--- (1980b) "Breeding Ecology of the Little Blue Heron on the West Coast of Florida." Condor 82:164-69.
Rodgers, J. A., Jr., and H. T. Smith (1995) "Little Blue Heron (Egretta caerulea)." In A. Poole and F. Gill, eds., The Birds of North America: Life Histories for the 21st Century, no. 145. Philadelphia: Academy of Natural Sciences; Washington, D.C.: American Ornithologists' Union.
Telfair, R. C., II (1994) "Cattle Egret (Bubulcus ibis)." In A. Poole and F. Gill, eds., The Birds of North America: Life Histories for the 21st Century, no. 113. Philadelphia: Academy of Natural Sciences; Washington, D.C.: American Ornithologists' Union.
* Werschkul, D. F. (1982) "Nesting Ecology of the Little Blue Heron: Promiscuous Behavior." Condor 84:381-84.
--- (1979) "Nestling Mortality and the Adaptive Significance of Early Locomotion in the Little Blue Heron." Auk 96:116-30.
{518}
 Rails nad Coots
Rails nad Coots
PUKEKO or PURPLE SWAMPHEN (Porphyrio porphyrio)
IDENTIFICATION: A large (nearly 20 inch) wading bird with bluish purple plumage, a red shield on its forehead, and red feet with long toes. DISTRIBUTION: From the western Mediterranean through Middle East, eastern and southern Africa, and throughout Australasia. HABITAT: Wetlands, especially swamps and marshy areas. STUDY AREA: Shakespear Regional Park, North Island, New Zealand; subspecies P.p. melanotus.
TASMANIAN NATIVE HEN (Tribonyx mortierii)
IDENTIFICATION: Similar to Pukeko, but flightless, and with grayish brown plumage, no red frontal shield, and shorter legs. DISTRIBUTION: Tasmania. HABITAT: Pasture, marshes, lakes, rivers. STUDY AREA: Hunting Ground, near Hobart, Tasmania.
DUSKY MOORHEN (Gallinula tenebrosa)
IDENTIFICATION: Similar to Pukeko, but with black plumage and shorter legs. DISTRIBUTION: Australia, New Guinea, Indonesia. HABITAT: Wetlands. STUDY AREAS: Sullivan's Creek and Gungahlin, near Canberra, Australia; subspecies
G.t. tenebrosa.
Social Organization
Pukeko are notable for their "communal" breeding system: they form stable groups of 4-14 birds during the mating season, usually with an equal number of males and females. All adult members (except nonbreeding "helpers," see below) generally mate with each other, and both sexes take turns incubating the eggs, which are often laid by several females in the same nest. Some birds pair off into (heterosexual) couples or remain single rather than forming communal groups, and outside the breeding season Pukeko usually live in flocks. Many Tasmanian Native Hens and Dusky Moorhens also live in (generally smaller) communal breeding groups that have various types of polygamous or promiscuous mating arrangements. Some Tasmanian Native Hens also form monogamous pair-bonds.
Description
Behavioral Expression: Both male and female Pukeko engage in homosexual courtship and copulation with members of their communal group; these activities {519}
are more common and more developed between female birds. Lesbian courtship consists of a sequence of three activities (which also occur during heterosexual interactions). The courtship begins with ALLOPREENING: one female approaches another and bows to her, initiating mutual preening (stylized stroking of each other's feathers with their bill). This is followed by COURTSHIP-FEEDING, in which the two females exchange ritual gifts of food (usually small leaves or shoots). Finally, copulation takes place: the courting bird approaches the other in a distinctive upright posture while making a nasal HUMMING CALL; her partner adopts a hunched posture and mounting takes place, often with full cloacal (genital) contact. Sometimes, the two females reciprocate, the bird who was mounted first then mounting the other. Male homosexuality usually involves copulation and sometimes allopreening, but not courtship-feeding. Unlike female same-sex interactions, sexual activity between males is often initiated by the bird that is mounted, who places himself in the hunched posture in front of the other male as an invitation to mount.
In Tasmanian Native Hens, homosexual copulations occur in both males (often younger birds) and females living in the same group, while only male Dusky Moorhens participate in same-sex mounting. In all three species, birds that engage in homosexual activities also undertake parental duties such as nest-building, egg laying, incubation, and care of chicks (whether their own or those of other members of their communal group). In fact, homosexual activity (like heterosexual activity) in female Pukeko is most frequent just before or during the period of egg laying. Because birds in Pukeko and Tasmanian Native Hen groups are often related to each other, at least some homosexual — as well as heterosexual — activity is incestuous (mostly between brothers in Tasmanian Native Hens).
Frequency: Homosexuality is common among Pukeko, occurring in nearly {520}
45 percent of all communal groups. In this species, 7 percent of all copulations are homosexual, while 24 percent of courtship-feedings and 59 percent of courtship allopreening interactions are same-sex. In Tasmanian Native Hens and Dusky Moorhens, homosexual copulations make up 1-2 percent of all matings.
Courtship and sexual activity between female Pukeko: "allopreening" (above), approach with "humming call" (middle), and mounting
Orientation: Many adult breeding Pukeko are probably bisexual, capable of engaging in both homosexual and heterosexual courtship and copulation. In some cases, birds alternate between same-sex and opposite-sex copulations in quick succession. Nonbreeding helpers, however, generally participate in neither heterosexual nor homosexual activity. Far fewer individuals participate in homosexual behavior in Tasmanian Native Hens and Dusky Moorhens, but those that do are probably also bisexual.
Nonreproductive and Alternative Heterosexualities
As described above, the most common social unit among these species of Rails is not the heterosexual pair or nuclear family, but the communal group. In some populations of Pukeko, each such group may include up to seven nonbreeding individuals who assist in parental duties. These "helpers" (offspring from previous years) may delay their own reproductive careers for up to three years (one or two years in Tasmanian Native Hens, where an average of 18 percent of adults are nonbreeders). Some physiological mechanisms may be involved in this breeding suppression, since Pukeko helpers often have underdeveloped reproductive organs. In addition to several forms of polygamy, a number of other mating and parenting arrangements are found in these groups. For example, although all birds in Tasmanian Native Hen groups usually mate with each other, sometimes only one male-female pair actually has reproductive copulations. This social system has been called GENETIC MONOGAMY (because only one couple breeds) within SOCIAL POLYGAMY (since multiple partners mate with each other). In addition, although most group members remain together for life, females occasionally "divorce" their mates and join a new group; in some cases, this may lead to a female parenting young that are not her own. Occasionally, she may behave aggressively toward these foster chicks, even expelling them from the group. More violent confrontations sometimes occur when chicks stray into neighboring territories, where they may be killed by the resident group members. However, chicks are sometimes also adopted by neighboring groups, in both Tasmanian Native Hens and Dusky Moorhens.
Among breeding Pukeko, up to five males may court and mount the same female in quick succession — in these cases, one or more of the males may not actually inseminate the female. In fact, the "success" rate for most heterosexual copulations is not high: between one-half and two-thirds do not involve full genital contact and so do not result in insemination. Females often resist heterosexual advances by refusing to allow males to mount them, pecking at males who do mount them, preventing genital contact by not raising their tails, and prematurely terminating mating attempts. Copulations also occur when females are not fertile — for example, long before egg laying begins — and some males mate repeatedly without ever fathering any offspring that year. Similarly, mounts without genital {521}
contact account for more than a third of Dusky Moorhen matings and 60 percent of Tasmanian Native Hen matings. A few of these are REVERSE copulations, in which the female mounts the male. Incestuous matings are also common in Pukeko and Tasmanian Native Hens. Nearly two-thirds of all Pukeko heterosexual copulations in some populations are between related individuals, including mother-son, father-daughter, and brother-sister matings. More than 40 percent of Tasmanian Native Hen breeding groups contain related adults that mate with each other (mostly siblings); in addition, about 10 percent of copulations involve parents mounting their own offspring, including young chicks.
Other Species
Stable homosexual pairs often form among Cranes (e.g., Grus spp.) in captivity, a group of birds that is closely related to Rails.
Sources
(* asterisked references discuss homosexuality/transgender)
* Archibald, G. W. (1974) "Methods for Breeding and Rearing Cranes in Captivity." International Zoo Yearbook 14:147-55.
Craig, J. L. (1990) "Pukeko: Different Approaches and Some Different Answers." In P. B. Stacey and W. D. Koenig, eds., Cooperative Breeding in Birds: Long-term Studies of Ecology and Behavior, pp. 385-412. Cambridge: Cambridge University Press.
* --- (1980) "Pair and Group Breeding Behavior of a Communal Gallinule, the Pukeko, Porphyrio p. melanotus." Animal Behavior 28:593-603.
--- (1977) "The Behavior of the Pukeko." New Zealand Journal of Zoology 4:413-33.
Craig, J. L., and I. G. Jamieson (1988) "Incestuous Mating in a Communal Bird: A Family Affair." American Naturalist 131:58-70.
* Derrickson, S. R., and J. W. Carpenter (1987) "Behavioral Management of Captive Cranes — Factors Influencing Propagation and Reintroduction." In G. W. Archibald and R. F. Pasquier, eds., Proceedings of the 1983 International Crane Workshop, pp. 493-511. Baraboo, Wis.: International Crane Foundation.
Garnett, S. T. (1980) "The Social Organization of the Dusky Moorhen, Gallinula tenebrosa Gould (Aves: Rallidae)." Australian Wildlife Research 7:103-12.
* --- (1978) "The Behavior Patterns of the Dusky Moorhen, Gallinula tenebrosa Gould (Aves: Rallidae)." Australian Wildlife Research 5:363-84.
Gibbs, H. L., A. W. Goldizen, C. Bullough, and A. R. Goldizen (1994) "Parentage Analysis of Multi-Male Social Groups of Tasmanian Native Hens (Tribonyx mortierii): Genetic Evidence for Monogamy and Polyandry." Behavioral Ecology and Sociobiology 35:363-71.
Goldizen, A. W., A. R. Goldizen, and T. Devlin (1993) "Unstable Social Structure Associated with a Population Crash in the Tasmanian Native Hen, Tribonyx mortierii." Animal Behavior 46:1013-16.
Goldizen, A. W., A. R. Goldizen, D. A. Putland, D. M. Lambert, C. D. Millar, and J. C. Buchan (1998) "'Wife-sharing' in the Tasmanian Native Hen (Gallinula mortierii): Is It Caused By a Male-biased Sex Ratio?" Auk 115:528-32.
* Jamieson, I. G., and J. L. Craig (1987a) "Male-Male and Female-Female Courtship and Copulation Behavior in a Communally Breeding Bird." Animal Behavior 35:1251-53.
--- (1987b) "Dominance and Mating in a Communal Polygynandrous Bird: Cooperation or Indifference Towards Mating Competitors?" Ethology 75:317-27.
Jamieson, I. G., J. S. Quinn, P. A. Rose, and B. N. White (1994) "Shared Paternity Among Non-Relatives Is a Result of an Egalitarian Mating System in a Communally Breeding Bird, the Pukeko." Proceedings of the Royal Society of London, Series B 257:271-77.
* Lambert, D. M., C. D. Millar, K. Jack, S. Anderson, and J. L. Craig (1994) "Single- and Multilocus DNA Fingerprinting of Communally Breeding Pukeko: Do Copulations or Dominance Ensure Reproductive Success?" Proceedings of the National Academy of Sciences 91:9641-45.
* Ridpath, M. G. (1993) "Tasmanian Native-hen, Tribonyx mortierii." In S. Marchant and P. J. Higgins, eds., Handbook of Australian, New Zealand, and Antarctic Birds, vol. 2, pp. 615-24. Melbourne: Oxford University Press. {522}
* --- (1972) "The Tasmanian Native Hen, Tribonyx mortierii. I. Patterns of Behavior. II. The Individual, the Group, and the Population." CSIRO Wildlife Research 17:1-90.
* Swengel, S. R., G. W. Archibald, D. H. Ellis, and D. G. Smith (1996) "Behavior Management." In D. H. Ellis, G. F. Gee, and C. M. Mirande, eds., Cranes: Their Biology, Husbandry, and Conservation, pp. 105-22. Washington, D.C.: National Biological Service; Baraboo, Wis.: International Crane Foundation.
 Other Waders
Other Waders
HAMMERHEAD (Scopus umbretta)
IDENTIFICATION: A medium-sized brown, storklike bird with a prominent crest almost as long as its bill. DISTRIBUTION: Throughout tropical Africa from Senegal to east and south Africa; Madagascar. HABITAT: Wetlands, including savanna and woodland near water. STUDY AREAS: Near Niono, Mali; Karen and Nairobi, Kenya.
Social Organization
Hammerheads are usually found in pairs or groups of 8-10 individuals, though larger groups of up to 50 birds may also congregate. The mating system is believed to involve monogamous pair-bonds. Couples build extraordinarily large domed nests, often several on the same territory.
Description
Behavioral Expression: Hammerheads engage in a striking group courtship ceremony that includes same-sex mounting. Gathering at dawn on open lawns, riverbanks, rocks, or in the trees, groups of 3-20 birds (including some mated pairs) begin calling in unison. Their calls consist of a series of loud, high-toned yips that develop into a rapid sequence of "purring" or trilling notes, often accompanied by wing-flapping. Two or more birds may then perform a NODDING DISPLAY to each other, in which the bill is rapidly bobbed up and down (sometimes still accompanied by the YIP-PURR chorus), or pairs of birds may run in circles side by side. The culmination of this spectacular display is a series of mountings, initiated when one bird runs up to another, drooping and flicking its wings while it raises and lowers its crest, or when one bird solicits another by crouching, cocking {523}
its tail, and partially opening its wings. One Hammerhead then hops onto the back of the other, similar to a heterosexual copulation — except that, in addition to males mounting females, males also mount other males, and females mount females as well as males. The mounting bird beats its wings and gives the YIP-PURR call, while the mounted bird presses its tail up against the lowered tail of the mounter (though no cloacal [genital] contact takes place). Sometimes the two birds face in opposite directions; reciprocal mounting (mounter and mountee exchanging positions, often several times in succession) also occurs, as do "pile-ups" of three or four birds all mounted on each other.
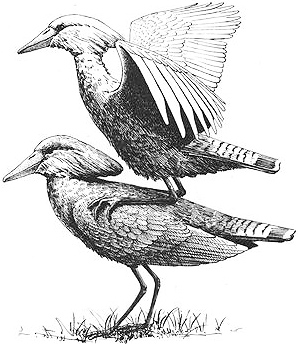
A female Hammerhead mounting another female
Frequency: Social courtship displays including ritualized same-sex mounting occur commonly in Hammerheads throughout the year. Each mounting session lasts for 10-40 minutes and may include dozens of mountings.
Orientation: All Hammerheads participate in group courtship displays, and most birds are probably involved in both same-sex and opposite-sex mountings.
Nonreproductive and Alternative Heterosexualities
As noted above, nonprocreative heterosexual activity (REVERSE mounting, as well as male-female mounts without cloacal contact) is common in Hammerheads. In addition, a significant percentage of heterosexual pairs may be nonbreeders. In some populations, as many as three-quarters of nests go unused (although some of these are "extra" nests built by breeding pairs), and couples may forgo breeding for four or more years at a time.
Other Species
Same-sex pairing occurs in semiwild White Storks (Ciconia ciconia) in some European populations, and individuals form homosexual pairs even when numerous opposite-sex partners are available. Same-sex pairs are able to successfully incubate, hatch, and raise foster young.
Sources
(* asterisked references discuss homosexuality/transgender)
* Brown, L. H. (1982) "Scopidae, Hamerkop." In L. H. Brown, E. K. Urban, and K. Newman, eds., The Birds of Africa, vol. 1, pp. 168-72. London and New York: Academic Press.
* Campbell, K. (1983) "Hammerkops." E.A.N.H.S. (East Africa Natural History Society) Bulletin (January-April): 11. 1. {524}
Cheke, A. S. (1968) "Copulation in the Hammerkop Scopus umbretta." Ibis 110:201-3.
* Elliott, A. (1992) "Scopidae (Hamerkop)." In J. del Hoyo, A. Elliott, and J. Sargatal, eds., Handbook of the Birds of the World, vol. 1, Ostrich to Ducks, pp. 430-35. Barcelona: Lynx Edicions.
Goodfellow, C. F. (1958) "Display in the Hamerkop, Scopus umbretta." Ostrich 29:1-4.
Kahl, M. P. (1967) "Observations on the Behavior of the Hamerkop Scopus umbretta in Uganda." Ibis 109:25-32.
* King, C. E. (1990) "Reproductive Management of the Oriental White Stork Ciconia boyciana in Captivity." International Zoo Yearbook 29:85-90.
Stowell, R. F. (1954) "A Note on the Behavior of Scopus umbretta." Ibis 96:150-51.
Wilson, R. T., and M. P. Wilson (1984) "Breeding Biology of the Hamerkop in Central Mali." In J. Ledger, ed., Proceedings of the 5th Pan-African Ornithological Congress, pp. 855-65. Johannesburg: Southern African Ornithological Society.
 Other Waders
Other Waders
FLAMINGO (Phoenicopterus ruber)
IDENTIFICATION: The largest flamingo species (4-5 feet tall) with plumage ranging from pale whitish pink to bright orange-pink. DISTRIBUTION: The Mediterranean, sub-Saharan Africa, western Asia, Caribbean and Galapagos Islands, temperate South America. HABITAT: Shallow lakes, lagoons, mudflats, salt pans. STUDY AREAS: Zoo Atlanta, Georgia; Audubon Park Zoo, New Orleans, Louisiana; Chester Zoo, United Kingdom; Rotterdam Zoo, the Netherlands; Basel Zoo, Switzerland; subspecies P.r. roseus, the Greater Flamingo; P.r. ruber, the Caribbean Flamingo; and P.r. chilensis, the Chilean Flamingo.
Social Organization
Flamingos are extremely gregarious birds, often congregating in enormous groups numbering in the tens of thousands. During the mating season pair-bonds are formed and the birds nest in large colonies.
Description
Behavioral Expression: Flamingos form both male and female homosexual pairs. The bond is similar to that of a heterosexual mated pair and is expressed through shared activities, such as feeding and traveling together, calling in {525}
unison, helping each other in aggressive encounters against other birds, and sleeping side by side. The pair-bond is also reinforced through a number of stylized displays, such as ritual preening and feeding, as well as standing in an alert posture (with a graceful S-curve in the neck) in front of each other. Partners may even engage in mounting and copulations with one another; full genital contact occurs in matings between females, but not usually between males. Early in the breeding season, single male Flamingos also sometimes pursue other males in an attempt to mount them; this is known as DRIVING. Single males seeking male partners have even been known to harass heterosexual pairs, following them around and disrupting their copulations and incubation shifts in an attempt to gain access to the male.
Once formed, homosexual pair-bonds are strong and may persist from one breeding season to the next. Most pairings are monogamous; however, in some male pairs the partners also attempt to mount other birds (usually incubating birds of either sex). Sometimes two males and a female will even form a TRIAD in which the two males are bonded or sexually interested in each other as much, if not more so, than they are in the female. Homosexual partners sometimes also build nests together; in the case of male pairs, the nest (a pedestal-shaped mud platform) may become exceptionally large because of the contributions of both partners. Some male pairs, rather than building their own nest, "steal" or take over the nest of a heterosexual pair, occasionally breaking eggs in the process (this behavior also occurs between heterosexual pairs).
Homosexual couples often engage in parenting behavior. Male pairs incubate, hatch, and successfully raise foster chicks (for example, from a nest they have taken over, or from eggs supplied in captivity). Described as "model" parents, male partners may even "nurse" their chick. Flamingo parents (of either sex) typically feed their chicks a blood-red "milk" produced in their crops, and both males in homosexual couples feed their chicks with this crop milk. Some male pairs, however, do not attempt to acquire eggs, even if they have their own nest, while others do not appear to be interested in parenting at all, since they may roll the egg out of a nest they have acquired. Female pairs take turns incubating eggs on their nest; such eggs may be infertile, having been laid by the females themselves rather than acquired from another nest. As in heterosexual pairs, variation exists in the amount of incubation time contributed by each partner. Some share incubation {526}
duties equally, while in other pairs, one female puts in more incubation shifts than the other. Overall, though, females in lesbian pairs contribute about five to six incubation shifts each, which is comparable to the average for heterosexual partners.

Two pair-bonded male Flamingos feeding crop milk to their foster chick
and tending it
Frequency. In most captive populations with same-sex couples, about 5-6 percent of pairs are homosexual, although some populations have more than a quarter same-sex couples. Although homosexual pairs have not yet been observed in the wild, oversize nests similar to those built by male pairs are found in most colonies and may in fact belong to homosexual pairs (especially considering that most field studies have not systematically and unambiguously determined the sexes of all paired birds).
Orientation: Overall, only a fraction of the population is involved in same-sex pairing. Among these, some individuals have no prior heterosexual experience. However, other Flamingos in homosexual pairs were previously members of a heterosexual pair (and vice versa). Some males in same-sex pairs also occasionally attempt to mount other birds, including females. These are both examples of different types of bisexuality (sequential and simultaneous). In populations with more males than females (or vice versa), many birds remain single rather than forming same-sex pair-bonds, perhaps indicating more of a heterosexual orientation on their part.
Nonreproductive and Alternative Heterosexualities
Although the standard social unit in Flamingos is the breeding monogamous pair, a number of alternative heterosexual pairing and family arrangements occur. Trios or triads — one male with two females or one female with two males — are fairly common (at least in zoos). Typically all three birds share incubation and chick-raising duties (although no same-sex bonding occurs); in trios with two females, there may be two separate nests, or both females may share a nest. Flamingo pairs also sometimes engage in nonreproductive copulations, mating far in advance of the female's fertile (or the male's sperm-producing) period. Many mated pairs are nonmonogamous, with both males and females seeking copulations with outside partners. In one zoo population, 47 percent of the females and 79 percent of the males participated in such "infidelity," and about 8 percent of all copulations were with outside partners; at another zoo, 25-60 percent of all pairs were nonmonogamous. Furthermore, divorce is extremely common in wild Flamingos: nearly all birds change partners between breeding seasons, and about 30 percent of males even switch mates during the season (in contrast, most pairings in zoos are long-lived).
Once chicks are hatched, a number of social systems are available to relieve the biological mother and father of some of their parenting duties. For example, nonbreeding birds sometimes produce crop milk and "nurse" other birds' chicks or else act as foster feeders for orphaned chicks. In addition, as they get older, Flamingo chicks typically gather into large nursery groups or
CRÈCHES, which may contain several thousand youngsters. These groups provide them with safety in the absence of direct parental supervision, and adults also sometimes feed youngsters other {527}
than their own in these creches. Chicks are often forced into creches as a result of attacks from adult birds, including their own parents, and creches may therefore also provide refuge from aggression by other Flamingos. Breeding in wild Flamingos can be irregular, with entire colonies sometimes forgoing reproduction for three or four years at a time — one colony in France failed to produce chicks for 13 out of 34 years (38 percent of the time). In addition, even if breeding is undertaken, it may be abruptly halted, with all or a large portion of the colony — often as many as half of all pairs — abandoning their eggs. Usually it is the female who initiates desertion of a nest.
Other Species
Homosexual pairs occur among both male and female Lesser Flamingos (Phoeniconaias minor) in captivity, including nest-building and (in females) egg-laying. Both sexes participate in homosexual mounting, though only males generally achieve full genital contact. Males in same-sex pairs also sometimes chase and mount females in lesbian pairs, while the latter may mate with males to fertilize their eggs. Most females in homosexual pairs, however, show no interest in males. Pairs of female Scarlet Ibises (Eudocimus ruber) have also been observed in captive flocks, nesting together and sometimes laying fertile eggs.
Sources
(* asterisked references discuss homosexuality/transgender)
Allen, R. P. (1956) The Flamingos: Their Life History and Survival. National Audubon Society Research Report no. 5. New York: National Audubon Society.
* Alraun, R., and N. Hewston (1997) "Breeding the Lesser Flamingo Phoeniconaias minor," Avicultural Magazine 103:175-81.
Bildstein, K. L., C. B. Golden, and B. J. McCraith (1993) "Feeding Behavior, Aggression, and the Conservation Biology of Flamingos: Integrating Studies of Captive and Free-Ranging Birds." American Zoologist 33:117-25.
Cezilly, F. (1993) "Nest Desertion in the Greater Flamingo, Phoenicopterus ruber roseus." Animal Behavior 45:1038-40.
Cezilly, F., and A. R. Johnson (1995) "Re-Mating Between and Within Breeding Seasons in the Greater Flamingo Phoenicopterus ruber roseus." Ibis 137:543-46.
* Elbin, S. B., and A. M. Lyles (1994) "Managing Colonial Waterbirds: the Scarlet Ibis Eudocimus ruber as a Model Species." International Zoo Yearbook 33:85-94.
Kahl, M. P. (1974) "Ritualized Displays." In J. Kear and N. Duplaix-Hall, eds., Flamingos, pp. 142-49. Berkhamsted, UK: T. and A. D. Poyser.
* King, C. E. (1996) Personal communication.
* --- (1994) "Management and Research Implications of Selected Behaviors in a Mixed Colony of Flamingos at Rotterdam Zoo." International Zoo Yearbook 33:103-13.
* --- (1993a) "Ondergeschoven Kinderen [Supposititious Children]." Dieren 10(4):116-19.
* --- (1993b) "Ongelukkige Flamingo Liefdes [Tales of Flamingo-Love Gone Awry]." Dieren 10(2):36-39.
Ogilvie, M., and C. Ogilvie (1989) Flamingos. Wolfeboro, N.H.: Alan Sutton.
* Shannon, P. (1985) "Flamingo Management at Audubon Park Zoo and the Benefits of Long-Term Research." In AAZPA Regional Conference Proceedings, pp. 226-36. Wheeling, W.Va.: AAZPA.
* Stevens, E. F. (1996) Personal communication.
--- (1991) "Flamingo Breeding: The Role of Group Displays." Zoo Biology 10:53-63.
* Studer-Thiersch, A. (1975) "Basle Zoo." In Kear and Duplaix-Hall, Flamingos, pp. 121-30.
Tourenq, C., A. R. Johnson, and A. Gallo (1995) "Adult Aggressiveness and Creching Behavior in the Greater Flamingo, Phoenicopterus ruber roseus." Colonial Waterbirds 18:216-21.
* Wilkinson, R. (1989) "Breeding and Management of Flamingos at Chester Zoo." Avicultural Magazine 95:51-61.
<<< ||||| >>>
 Geese
Geese


 Geese
Geese

 Swans
Swans

 Ducks
Ducks


 Other Ducks
Other Ducks
 Auks and Albatrosses
Auks and Albatrosses
 Cormorants
Cormorants
 Grebes
Grebes
 Herons
Herons Herons and Egrets
Herons and Egrets Rails nad Coots
Rails nad Coots
 Other Waders
Other Waders
 Other Waders
Other Waders